The Atom The beginning of the Atomic Theory

The Atom

The beginning of the Atomic Theory n Before the 19 th century n People thought an atom was a solid ball n Eugene Goldstein’s 1886 discovery that atoms had positive charges

1897 n J. J. Thomas discovered the electron which change theory drastically n His experiment was based on explaining the reason for a glowing material that was emitted from electric current passing a vacuum tube n He believed there were small parts of the atom getting ripped off n He was later proven right

1911 n Ernest n He Rutherford played with radioactive alpha particles n fired the particles at gold foil. n He found that while most of the particles passed right through the gold foil, a small number of particles passed through at an angle and some bounced straight back n He believed there were small particles in the middle of the atom that possessed positive charges which were surrounded by electrons
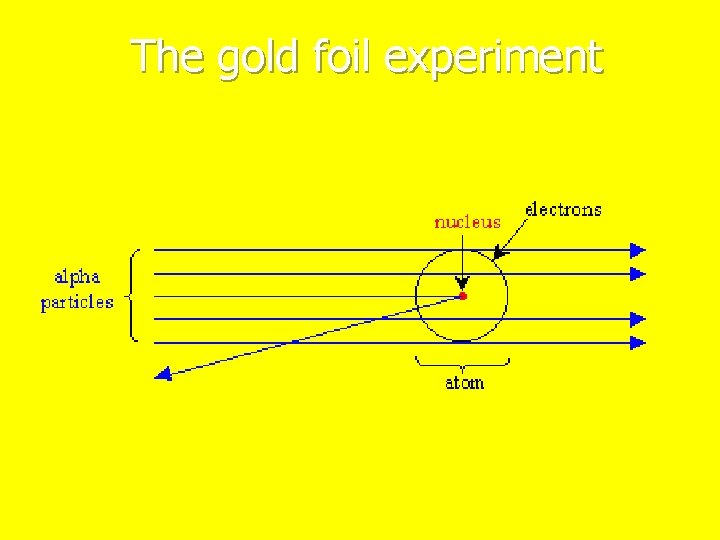
The gold foil experiment

1932, James Chadwick He discovered the third particle called the neutron n He theorized that something had to be in the nucleus to neutralize the charges of the electrons and protons n

Niels Bohr n This theory established the first description of the behavior of electrons on atoms. n Bohr’s theories led us to the modern structure of an atom that we use today

All matter is made up of atoms n There are three parts of an atom n. Proton n. Neutron n. Electron
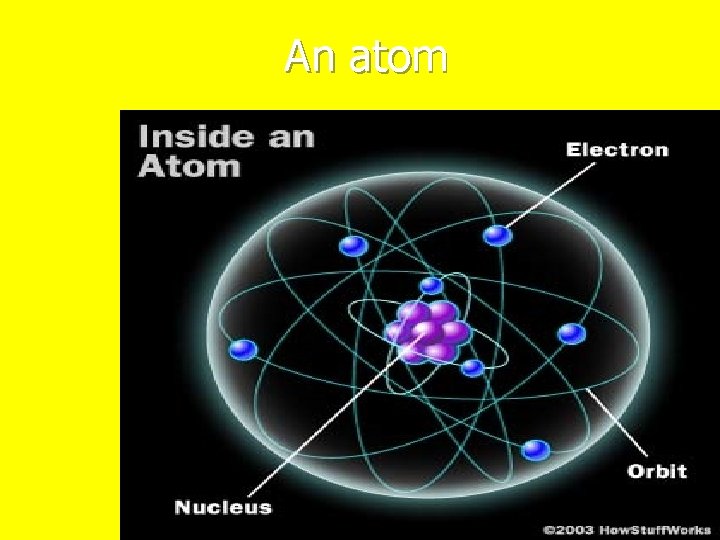
An atom
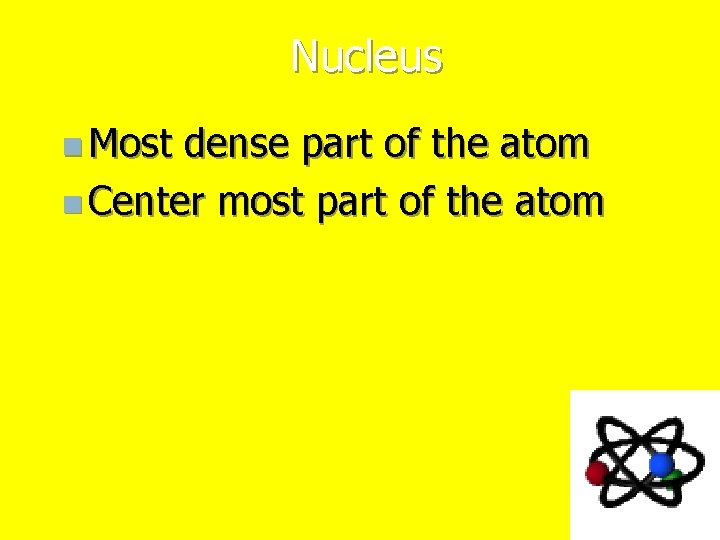
Nucleus n Most dense part of the atom n Center most part of the atom
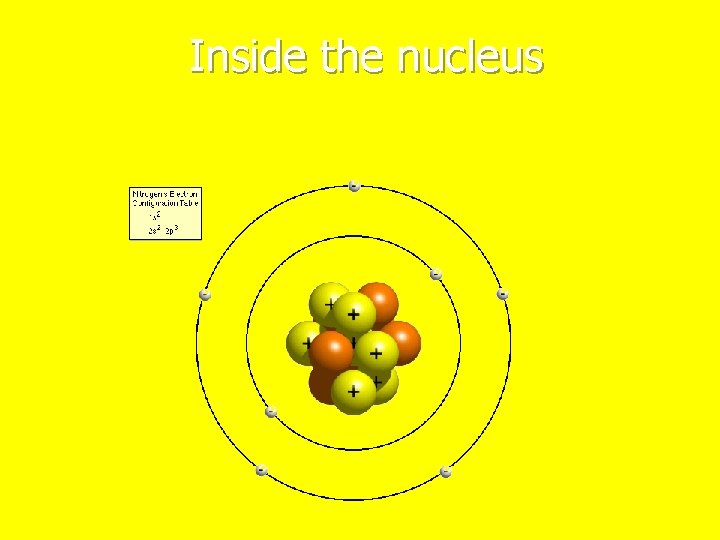
Inside the nucleus
Protons n They have a positive charge n Found inside the nucleus n Makes the difference between elements

Neutrons n They have a neutral charge or no charge n Found inside the nucleus

Electrons n They have a negative charge and is the smallest part n Found in the electron cloud n This is outside the nucleus n Electrons move fast around the nucleus and the position of an electron is hard to pin down n. The path of the electron is called an orbit

One atom is called an element n Lets look at water (H 2 O) n Water has 2 hydrogens and one oxygen n We have two elements that we are dealing with n. Hydrogen = 2 atoms of hydrogen n. Oxygen = 1 atom of oxygen

Isotopes n. They are atoms that have the right number of protons and electrons but differ in the amount of neutrons
Elements are arranged in a table called the periodic table n It provides you information about each element n Information like n. Mass n. How many protons n. Element’s symbols

Lets take a look at Carbon n Top number is the atomic number n C is the atomic symbol n Carbon is the element n 12. 011 is the mass 6 C Carbon 12. 011

What is what? n Atomic n is number the amount of protons n Atomic mass n average weight of all the isotopes of that atom n To find the mass of an element you just add the number of protons and neutrons
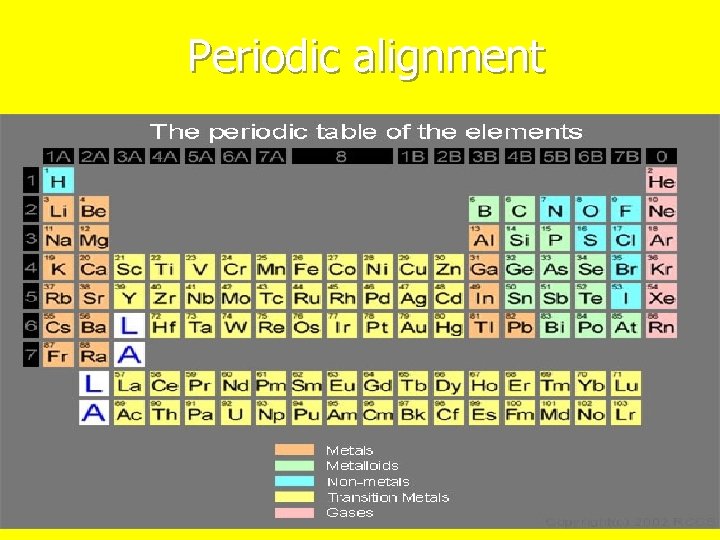
Periodic alignment

Rows or Periods 7 rows n 2 of the rows are pulled out to make the table easier to read n Properties gradually changes as you move across each row n. Become less reactive n

Columns or Families 18 groups n Properties in groups are similar but not identical n Some groups have special names n A 1 alkali metals n A 2 alkaline earth metals n A 3 – A 12 Transition metals n A 17 Halogens “salt forming” n A 18 Noble gases n

Properties of Metals n Solid at room temperature have 1 -3 electrons in their outer shell. n Lose their valence electrons easily n Good electrical conductors and heat conductors n Malleable n n can n Ductile n can n be beaten into thin sheets. be stretched into wire. Possess metallic luster

Nonmetals Usually have 4 -8 electrons in their outer shell n Gain or share valence electrons easily. n Poor conductors of heat and electricity. n Brittle - if a solid n Nonductile. n Do not possess metallic luster n Solids, liquids or gases at room temperature n
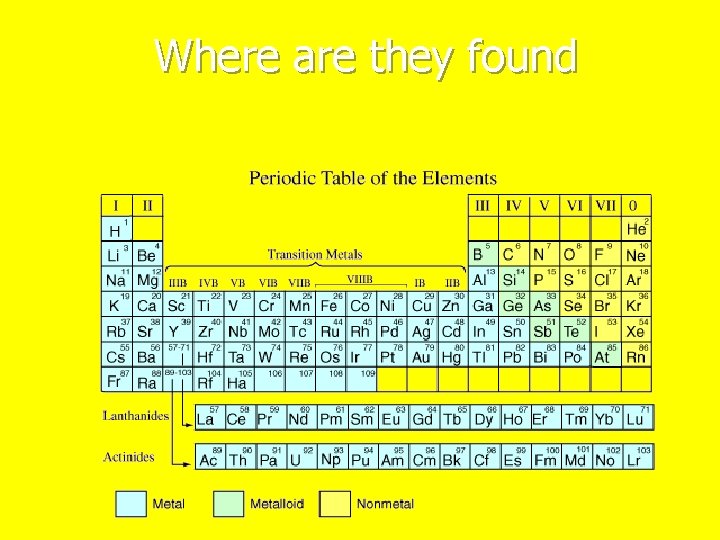
Where are they found
- Slides: 25