The Atom The Atom An atom consists of







- Slides: 32

The Atom

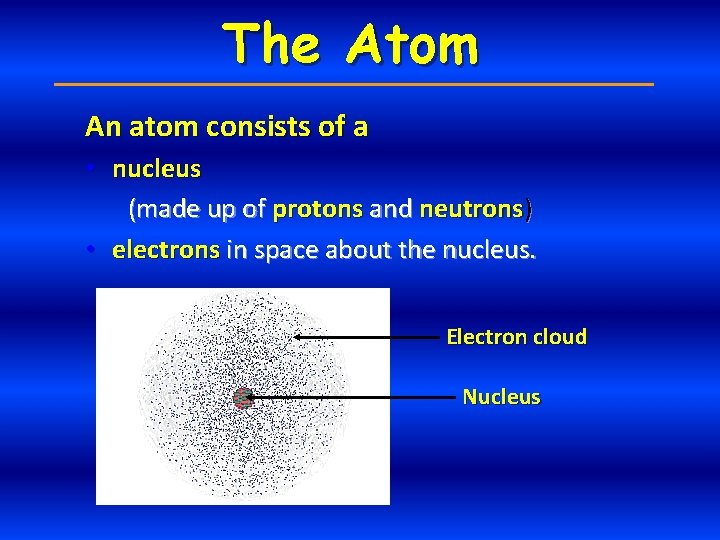
The Atom An atom consists of a • nucleus (made up of protons and neutrons) • electrons in space about the nucleus. Electron cloud Nucleus

Size of an Atom • http: //www. teachertube. com/video/power-of -ten-180460 A cube of sugar contains as many atoms as there are stars in the Universe!

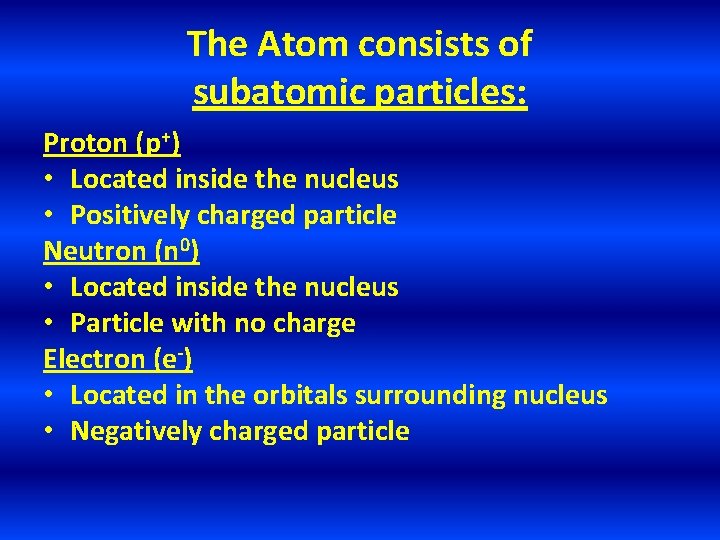
The Atom consists of subatomic particles: Proton (p+) • Located inside the nucleus • Positively charged particle Neutron (n 0) • Located inside the nucleus • Particle with no charge Electron (e-) • Located in the orbitals surrounding nucleus • Negatively charged particle

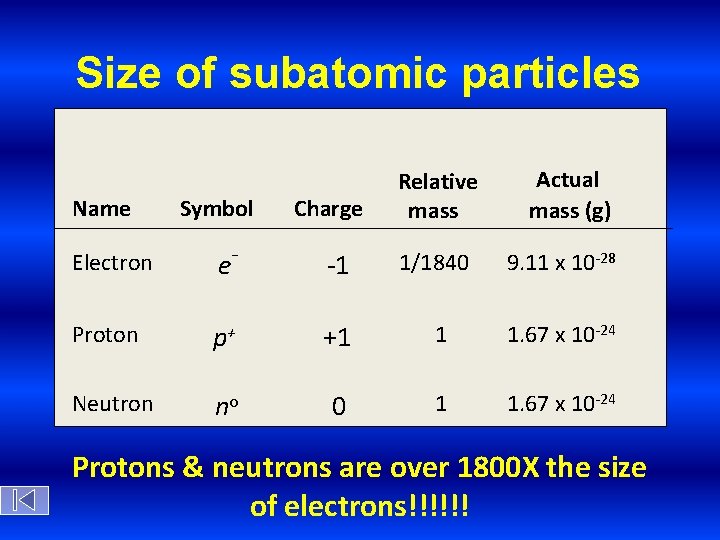
Size of subatomic particles Name Symbol Charge Relative mass Actual mass (g) Electron e- -1 Proton p+ +1 1 1. 67 x 10 -24 Neutron no 0 1 1. 67 x 10 -24 1/1840 9. 11 x 10 -28 Protons & neutrons are over 1800 X the size of electrons!!!!!!

The atom is mostly EMPTY SPACE Field Trip!!!!!!! Therefore… If the nucleus was the size of a tennis ball, the electron would be the size of a marble, and there would be about ½ mile in between the nucleus and the electron.


Counting the Pieces Atomic Number = number of protons # of protons determines kind of atom Protons are the identification piece to the element!!! The number of protons in an atom DO NOT CHANGE!!!! Atomic Number = number of electrons in a neutral atom Mass Number = the number of protons + neutrons California WEB

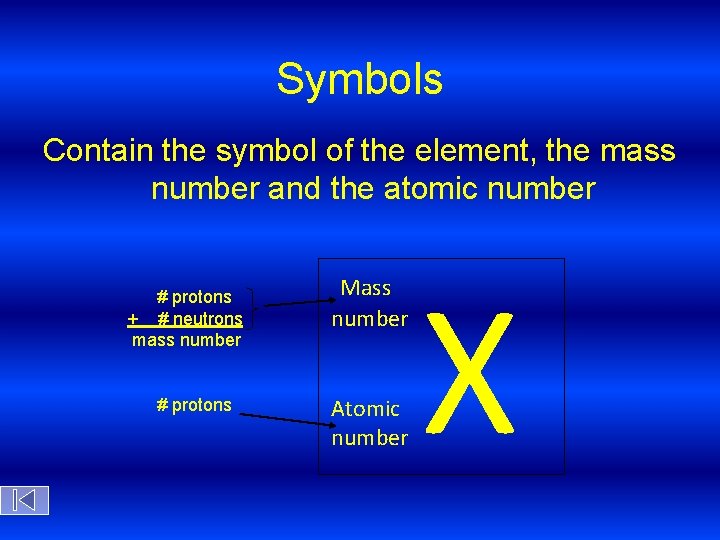
Symbols Contain the symbol of the element, the mass number and the atomic number # protons + # neutrons mass number # protons Mass number Atomic number X

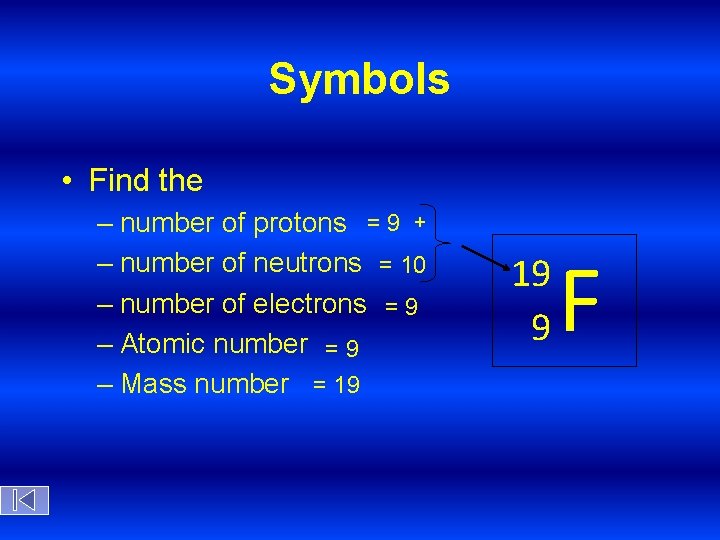
Symbols • Find the – number of protons = 9 + – number of neutrons = 10 – number of electrons = 9 – Atomic number = 9 – Mass number = 19 19 9 F

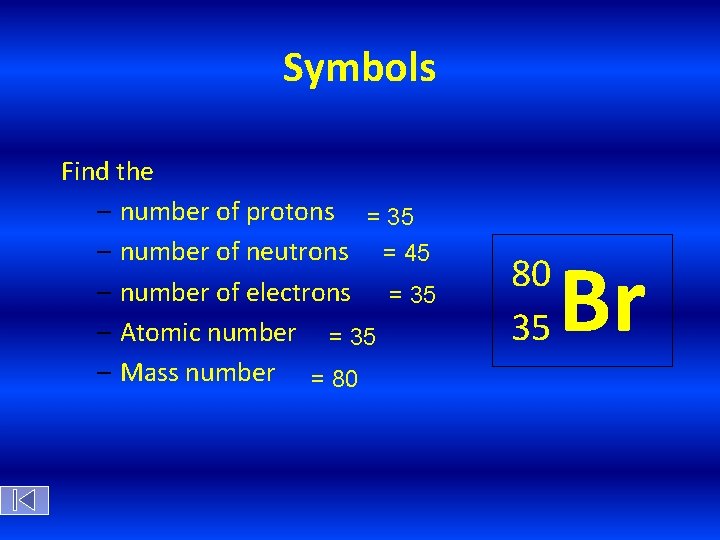
Symbols Find the – number of protons = 35 – number of neutrons = 45 – number of electrons = 35 – Atomic number = 35 – Mass number = 80 80 35 Br

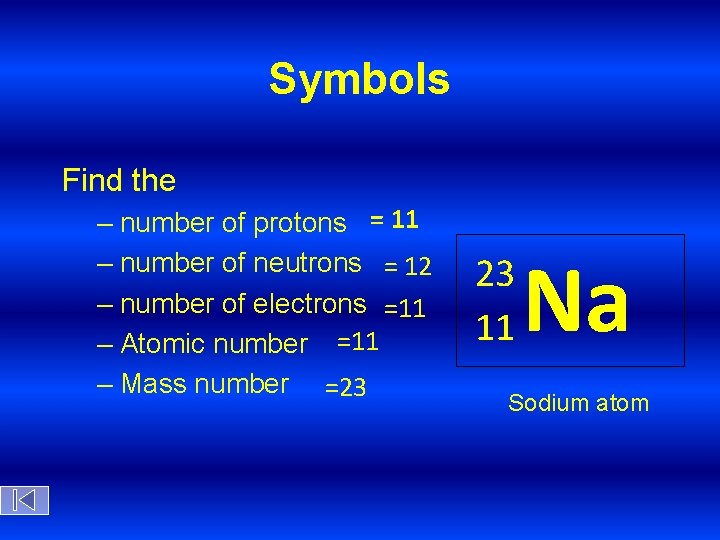
Symbols Find the – number of protons = 11 – number of neutrons = 12 – number of electrons =11 – Atomic number =11 – Mass number =23 23 11 Na Sodium atom

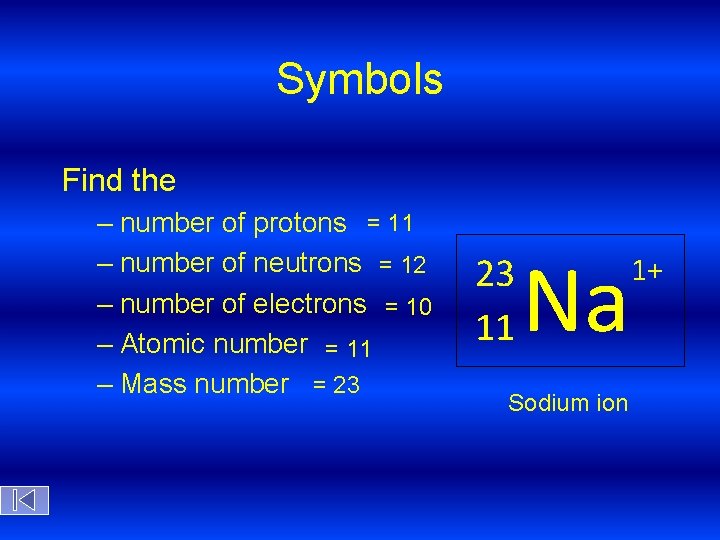
Symbols Find the – number of protons = 11 – number of neutrons = 12 – number of electrons = 10 – Atomic number = 11 – Mass number = 23 23 11 Na Sodium ion 1+

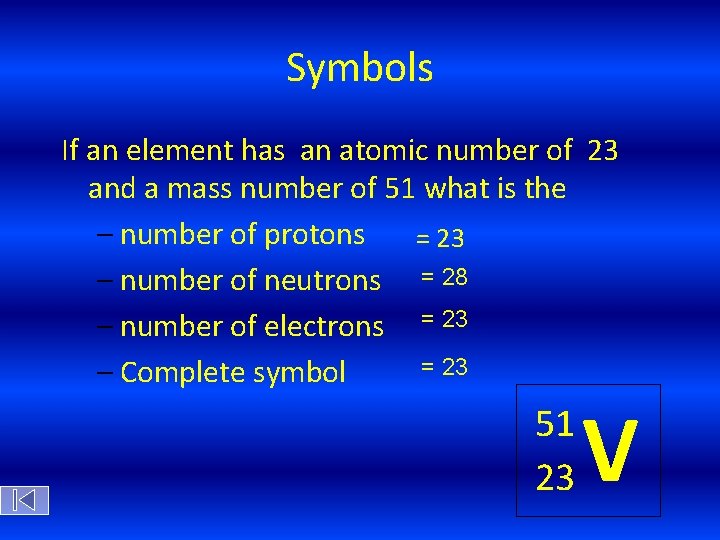
Symbols If an element has an atomic number of 23 and a mass number of 51 what is the – number of protons = 23 – number of neutrons = 28 – number of electrons = 23 – Complete symbol 51 23 V

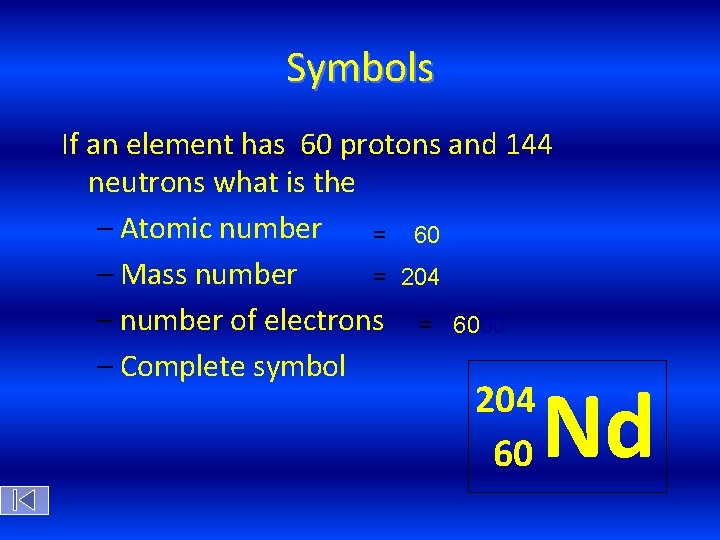
Symbols If an element has 60 protons and 144 neutrons what is the – Atomic number = 60 – Mass number = 204 – number of electrons = 6060 – Complete symbol 204 60 Nd

IONS • IONS are atoms or groups of atoms with a positive or negative charge. • Taking away an electron from an atom gives a CATION with a positive charge • Adding an electron to an atom gives an ANION with a negative charge. • To tell the difference between an atom and an ion, look to see if there is a charge in the superscript! Examples: Na+ Ca+2 I- O-2 Na Ca I O

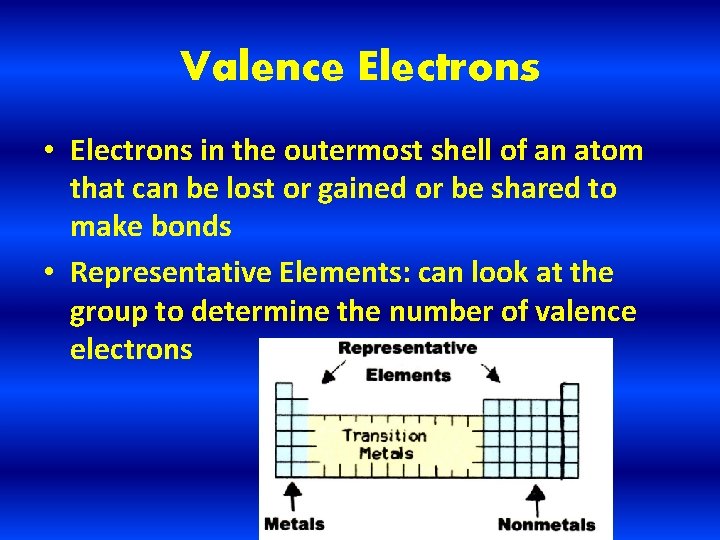
Valence Electrons • Electrons in the outermost shell of an atom that can be lost or gained or be shared to make bonds • Representative Elements: can look at the group to determine the number of valence electrons

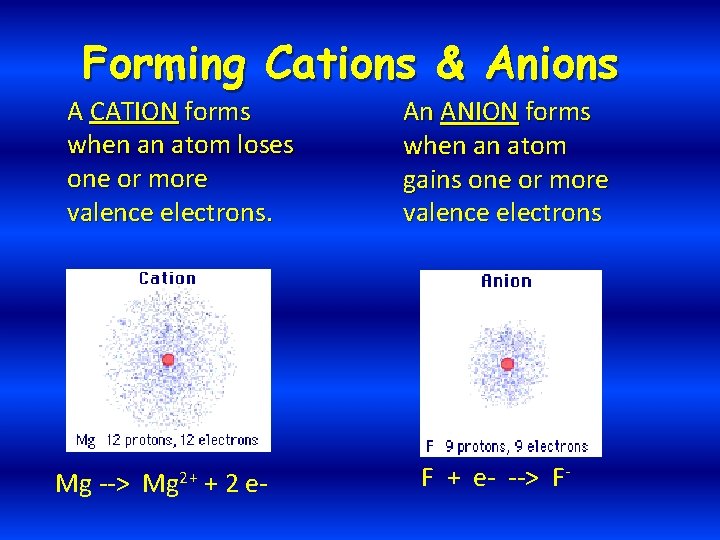
Forming Cations & Anions A CATION forms when an atom loses one or more valence electrons. Mg --> Mg 2+ + 2 e- An ANION forms when an atom gains one or more valence electrons F + e- --> F-

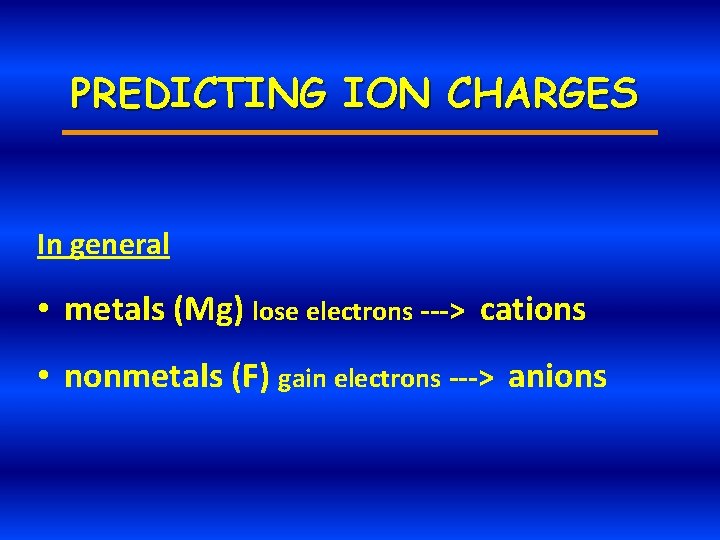
PREDICTING ION CHARGES In general • metals (Mg) lose electrons ---> cations • nonmetals (F) gain electrons ---> anions

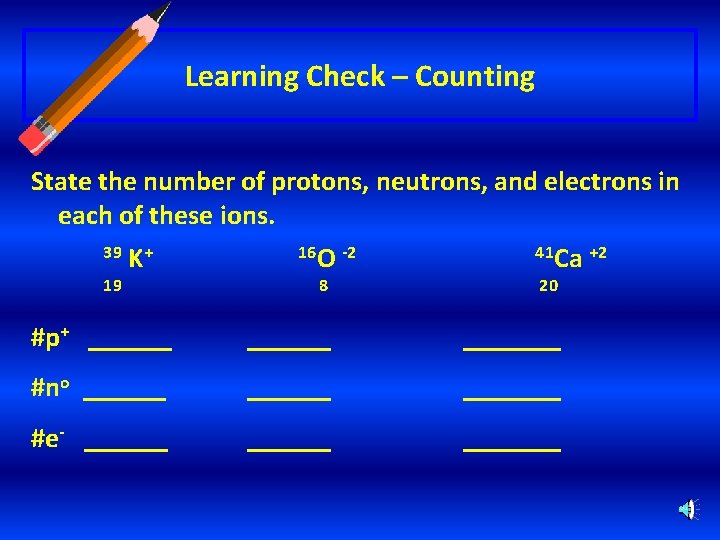
Learning Check – Counting State the number of protons, neutrons, and electrons in each of these ions. 39 19 K+ 16 O -2 8 41 Ca +2 20 #p+ _______ #no _______ #e- _______

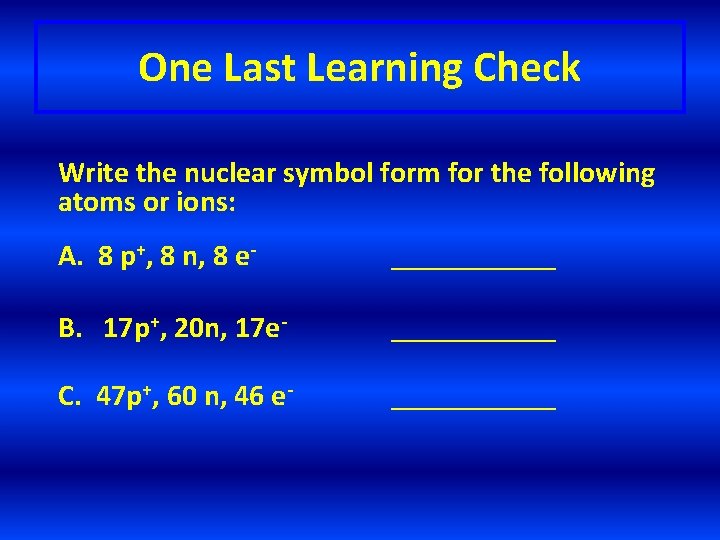
One Last Learning Check Write the nuclear symbol form for the following atoms or ions: A. 8 p+, 8 n, 8 e- ______ B. 17 p+, 20 n, 17 e- ______ C. 47 p+, 60 n, 46 e- ______

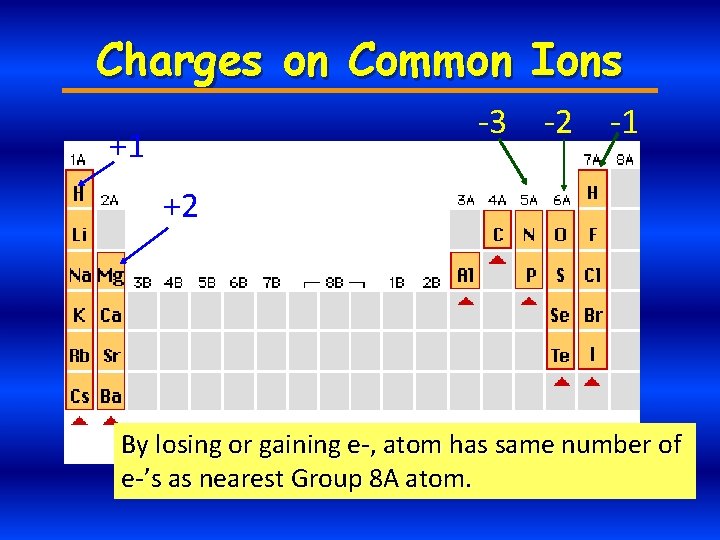
Charges on Common Ions -3 +1 -2 -1 +2 By losing or gaining e-, atom has same number of e-’s as nearest Group 8 A atom.

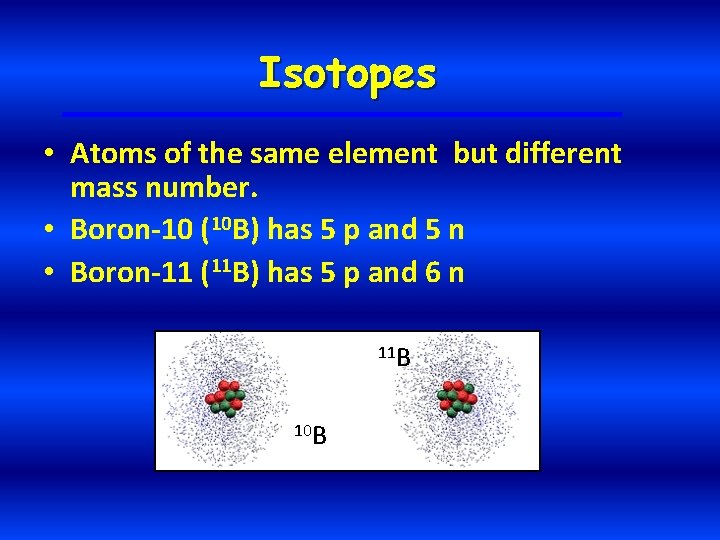
Isotopes • Atoms of the same element but different mass number. • Boron-10 (10 B) has 5 p and 5 n • Boron-11 (11 B) has 5 p and 6 n 11 B 10 B

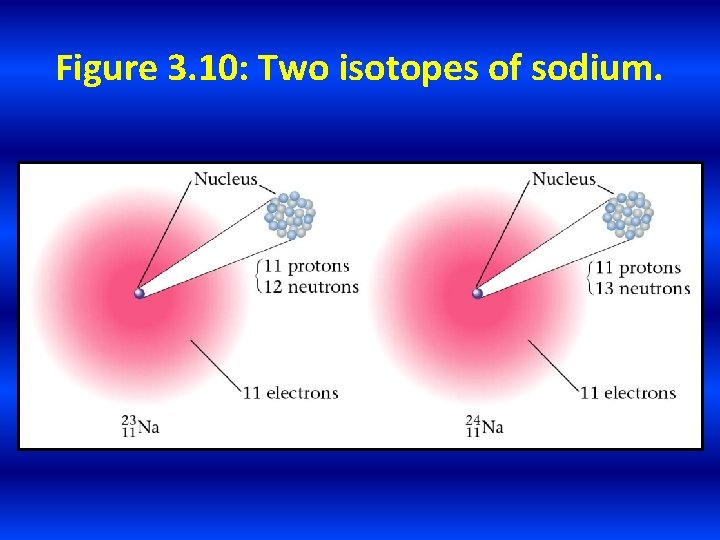
Figure 3. 10: Two isotopes of sodium.

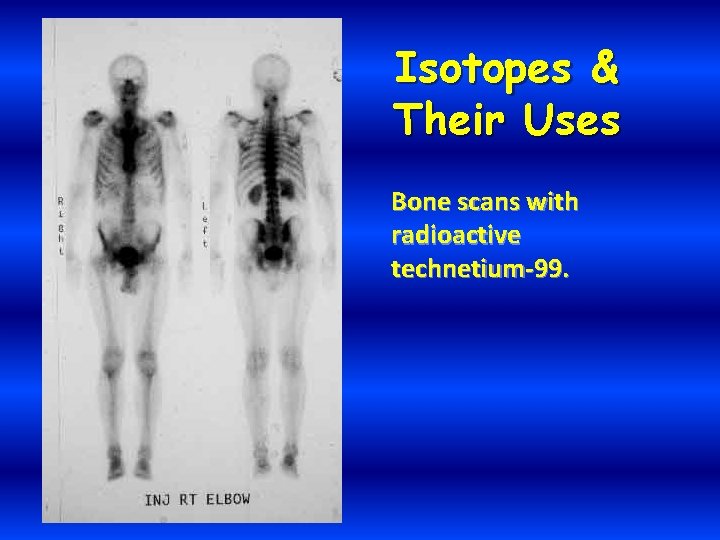
Isotopes & Their Uses Bone scans with radioactive technetium-99.

Isotopes & Their Uses The tritium (isotope of H) content of ground water is used to discover the source of the water, for example, in municipal water or the source of the steam from a volcano. (Note: Tritium is illegal for public use in the U. S. )

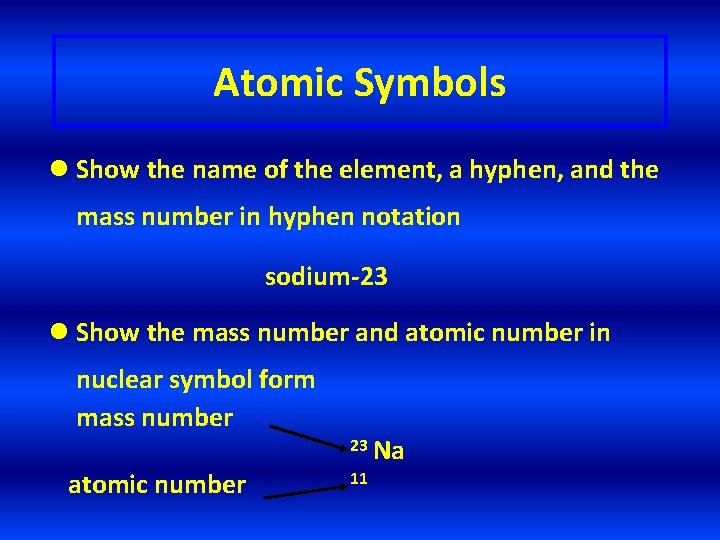
Atomic Symbols l Show the name of the element, a hyphen, and the mass number in hyphen notation sodium-23 l Show the mass number and atomic number in nuclear symbol form mass number atomic number 23 Na 11

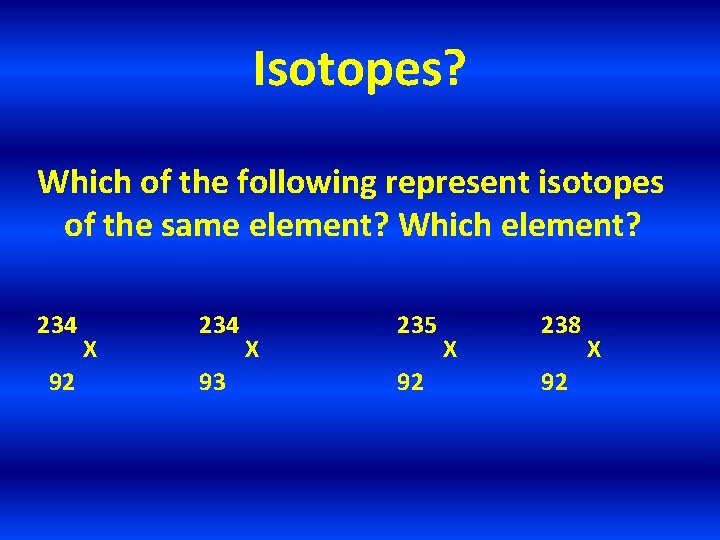
Isotopes? Which of the following represent isotopes of the same element? Which element? 234 92 X 234 93 X 235 92 X 238 92 X

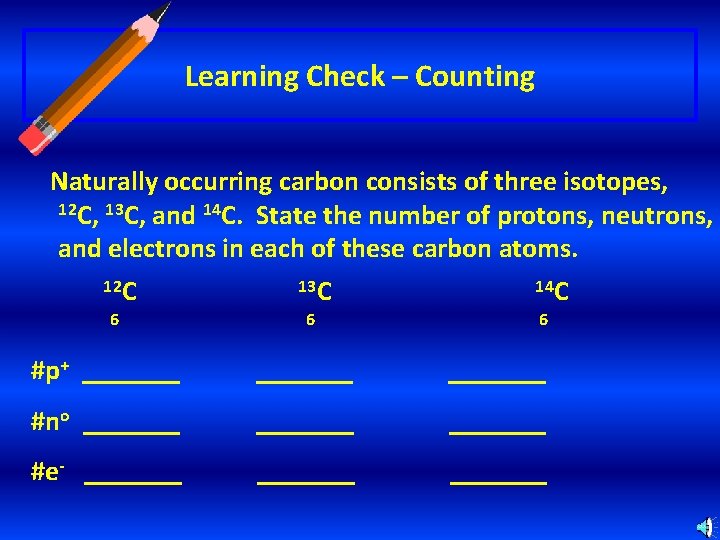
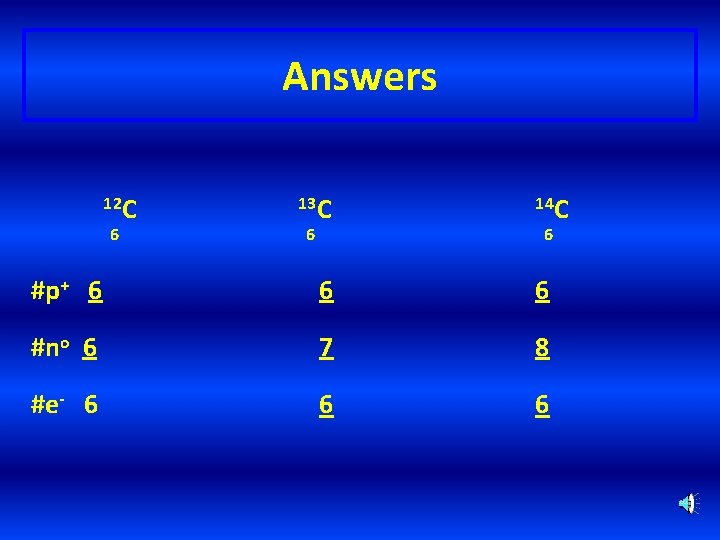
Learning Check – Counting Naturally occurring carbon consists of three isotopes, 12 C, 13 C, and 14 C. State the number of protons, neutrons, and electrons in each of these carbon atoms. 12 C 6 13 C 6 14 C 6 #p+ _______ #no _______ #e- _______

Answers 12 C 6 13 C 6 14 C 6 #p+ 6 6 6 #no 6 7 8 #e- 6 6 6

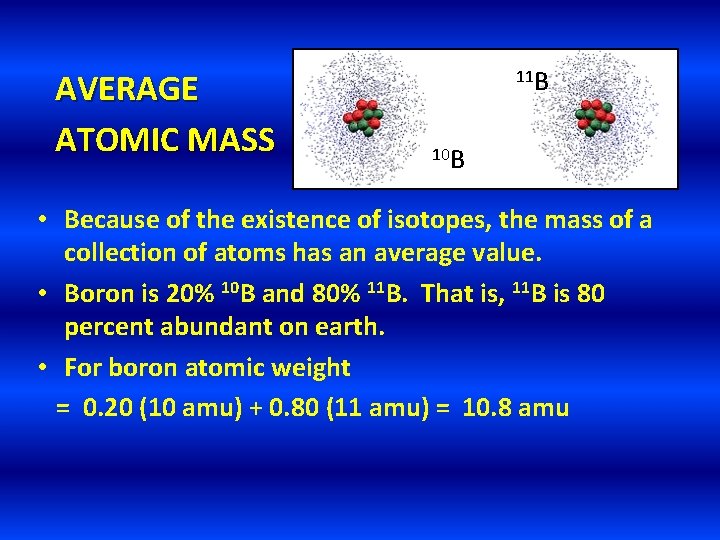
AVERAGE ATOMIC MASS 11 B 10 B • Because of the existence of isotopes, the mass of a collection of atoms has an average value. • Boron is 20% 10 B and 80% 11 B. That is, 11 B is 80 percent abundant on earth. • For boron atomic weight = 0. 20 (10 amu) + 0. 80 (11 amu) = 10. 8 amu

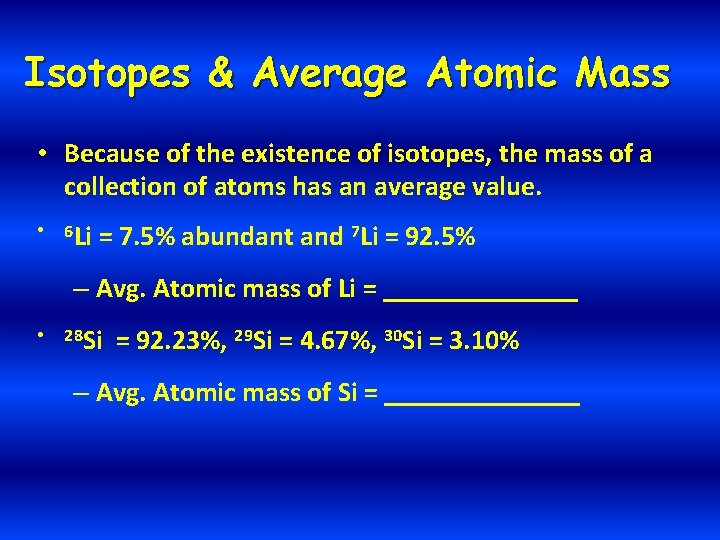
Isotopes & Average Atomic Mass • Because of the existence of isotopes, the mass of a collection of atoms has an average value. • 6 Li = 7. 5% abundant and 7 Li = 92. 5% – Avg. Atomic mass of Li = _______ • 28 Si = 92. 23%, 29 Si = 4. 67%, 30 Si = 3. 10% – Avg. Atomic mass of Si = _______