The Atom Science 9 Objectives By the end
The Atom Science 9

Objectives By the end of the lesson you should be able to: • Identify and describe three main parts of an atom • The relationship between atoms and elements • How to write chemical symbols • Briefly describe some common elements
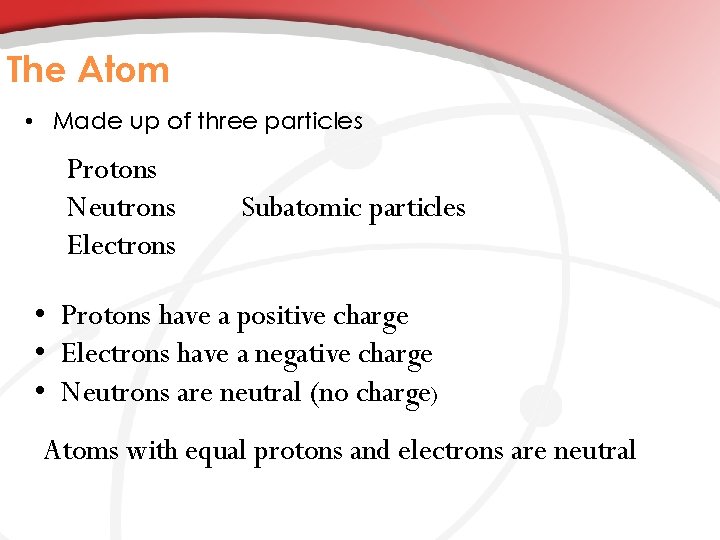
The Atom • Made up of three particles Protons Neutrons Electrons Subatomic particles • Protons have a positive charge • Electrons have a negative charge • Neutrons are neutral (no charge) Atoms with equal protons and electrons are neutral
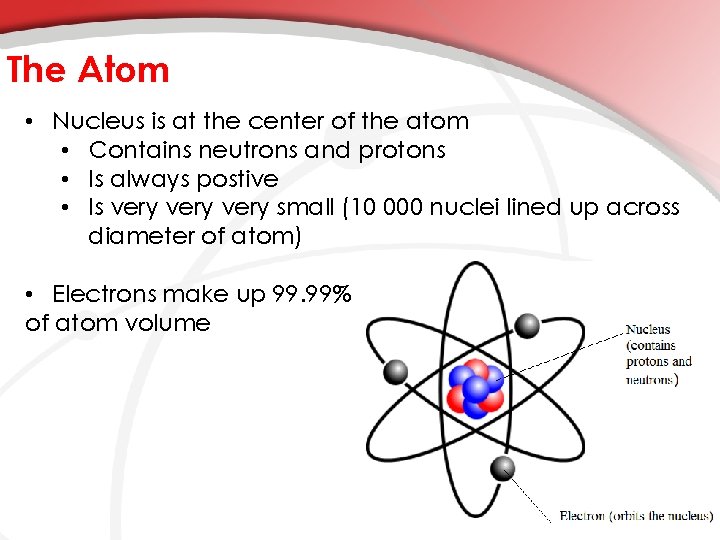
The Atom • Nucleus is at the center of the atom • Contains neutrons and protons • Is always postive • Is very small (10 000 nuclei lined up across diameter of atom) • Electrons make up 99. 99% of atom volume
Elements and Atoms • Elements cannot be broken down • Each element is made of one type of atom • Atoms differ in the number of protons, neutrons and electrons • All protons are identical • Protons in carbon are the same as protons in gold • All atoms of an element have the same number of protons • ONLY protons determine the type of element
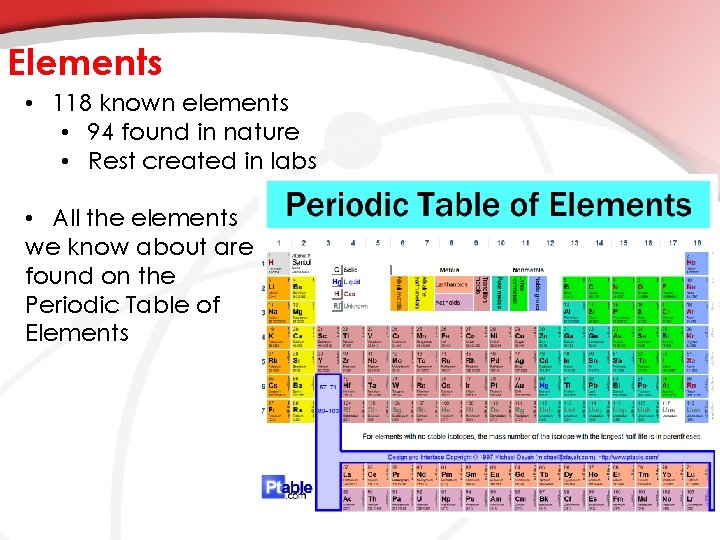
Elements • 118 known elements • 94 found in nature • Rest created in labs • All the elements we know about are found on the Periodic Table of Elements
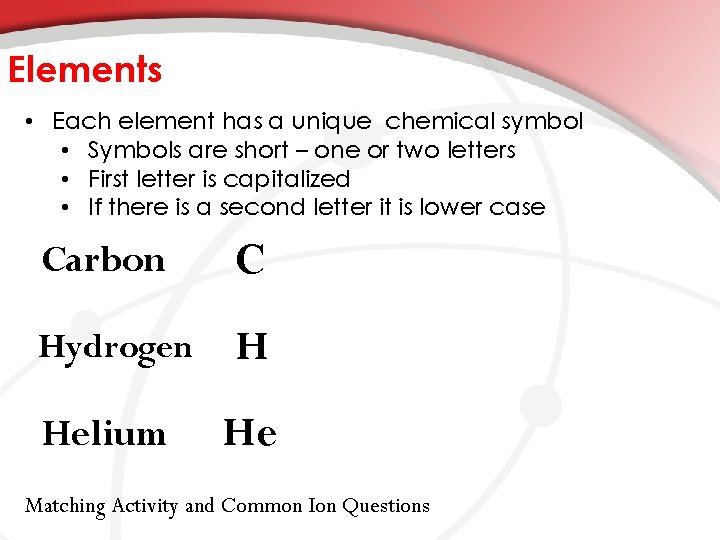
Elements • Each element has a unique chemical symbol • Symbols are short – one or two letters • First letter is capitalized • If there is a second letter it is lower case Carbon C Hydrogen H Helium He Matching Activity and Common Ion Questions
- Slides: 7