The Atom Basic Composition An atom is composed

The Atom
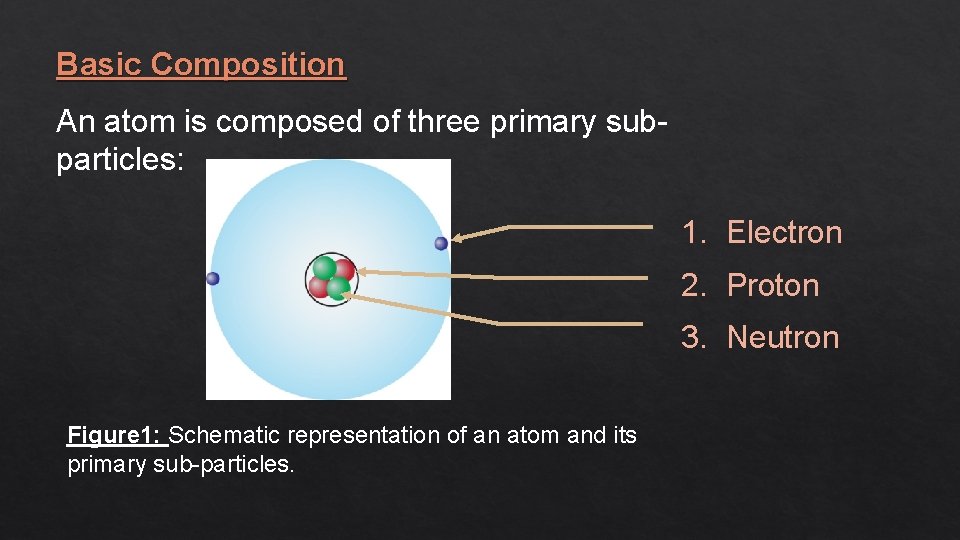
Basic Composition An atom is composed of three primary subparticles: 1. Electron 2. Proton 3. Neutron Figure 1: Schematic representation of an atom and its primary sub-particles.
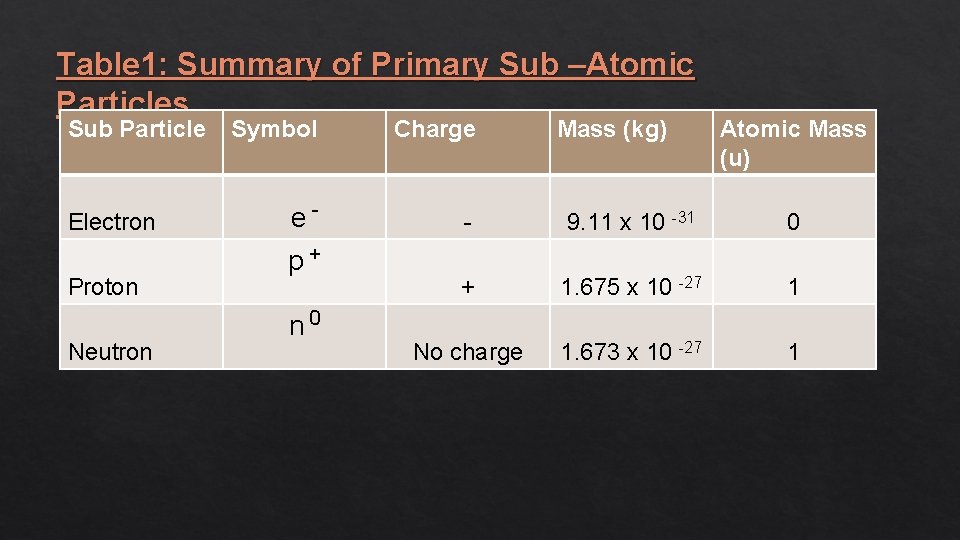
Table 1: Summary of Primary Sub –Atomic Particles Sub Particle Electron Proton Neutron Symbol Charge e- - 9. 11 x 10 -31 0 + 1. 675 x 10 -27 1 No charge 1. 673 x 10 -27 1 p+ n 0 Mass (kg) Atomic Mass (u)
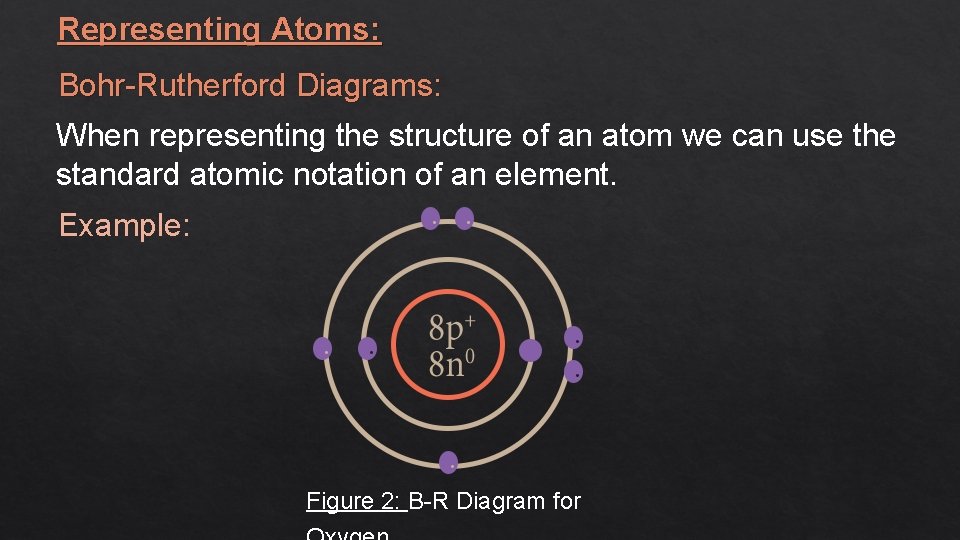
Representing Atoms: Bohr-Rutherford Diagrams: When representing the structure of an atom we can use the standard atomic notation of an element. Example: Figure 2: B-R Diagram for
Steps to Draw a Bohr-Rutherford Diagram: 1. Draw a circle to represent the nucleus. 2. Using the Standard Atomic Notation, indicate the number of protons and neutrons. Protons – The atomic number represents the number of protons present. Neutrons – Subtract the atomic number from the mass number.
Steps to Draw a Bohr-Rutherford Diagram: 3. Arrange the electrons by placing rings around the nucleus. (Each ring has a limit or capacity for the number of electrons allowed) i) Determine the number of rings by locating the period the element is located ii) Determine the number of electron in a ring by counting the number of elements in each period.
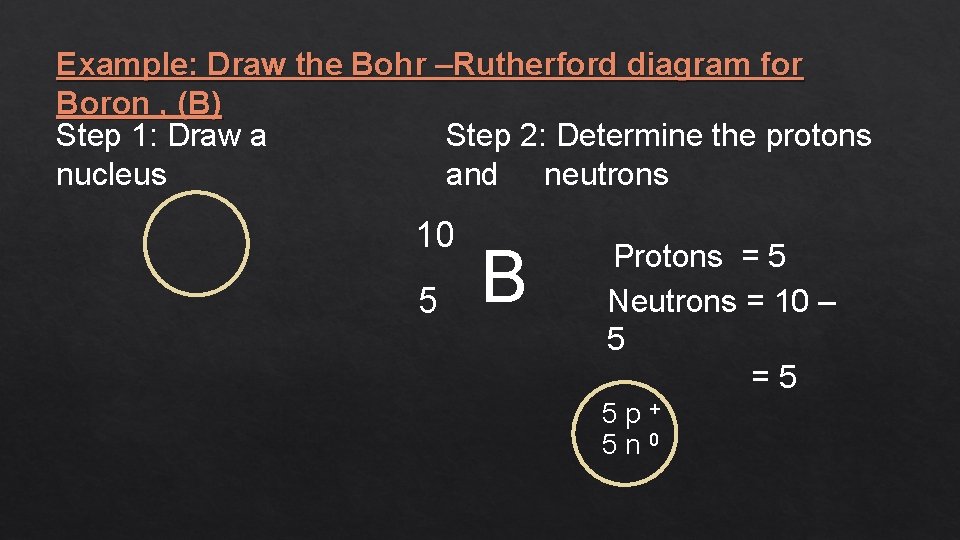
Example: Draw the Bohr –Rutherford diagram for Boron , (B) Step 2: Determine the protons Step 1: Draw a and neutrons nucleus 10 5 B Protons = 5 Neutrons = 10 – 5 =5 5 p+ 5 n 0
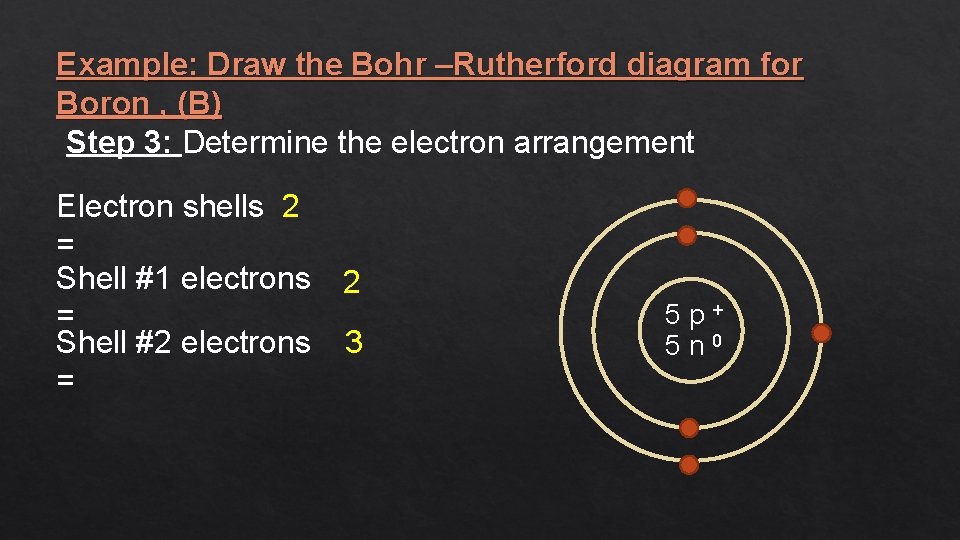
Example: Draw the Bohr –Rutherford diagram for Boron , (B) Step 3: Determine the electron arrangement Electron shells 2 = Shell #1 electrons = Shell #2 electrons = 2 3 5 p+ 5 n 0
- Slides: 8