THE ATOM Atomic Number How are atoms of

THE ATOM!

Atomic Number How are atoms of one element different from another? Elements contain different number of protons! # of protons = # of electrons ATOMIC NUMBER (Z) is the number of protons in the nucleus.

Determine the number of Protons Element Carbon Phosphorus Gold Protons 6 15 79 Atomic # Z 6 15 79
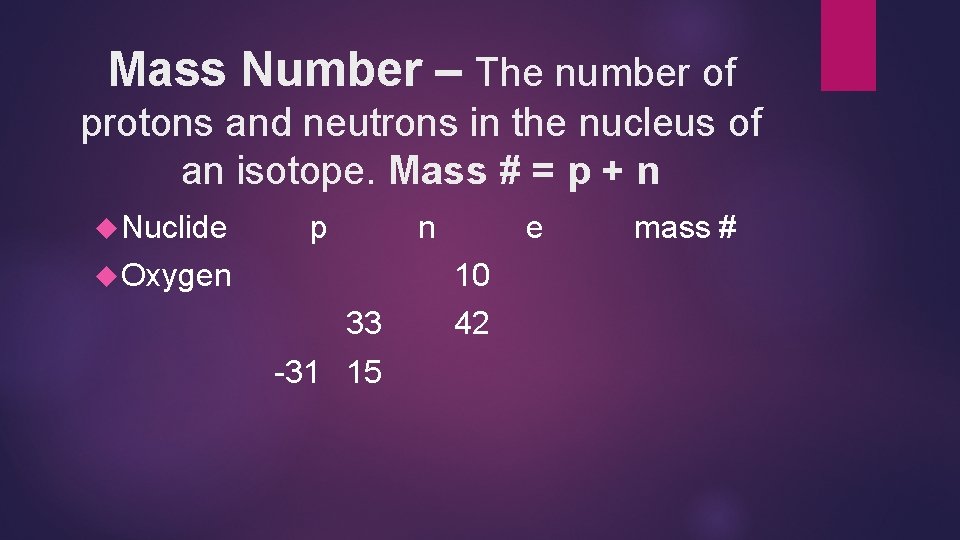
Mass Number – The number of protons and neutrons in the nucleus of an isotope. Mass # = p + n Nuclide p n Oxygen e 10 33 -31 15 42 mass #
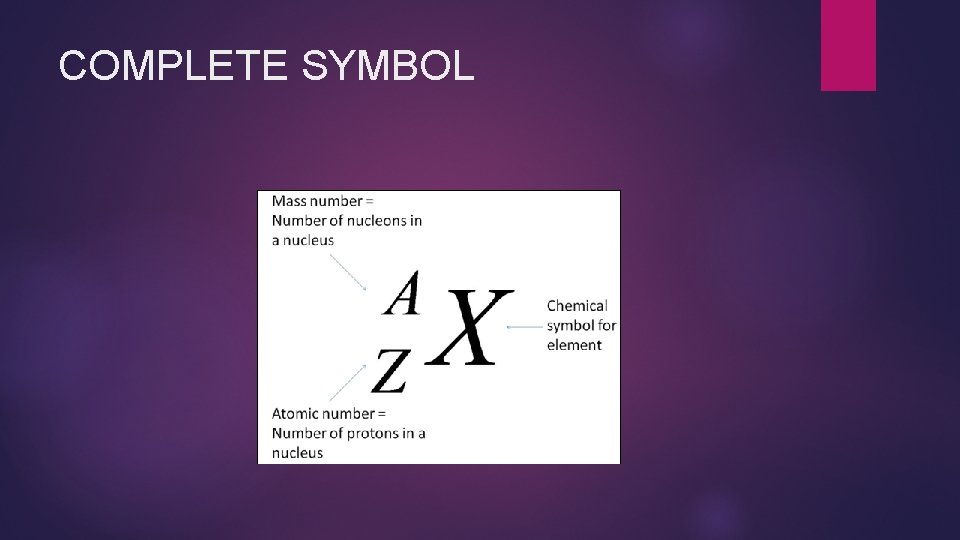
COMPLETE SYMBOL
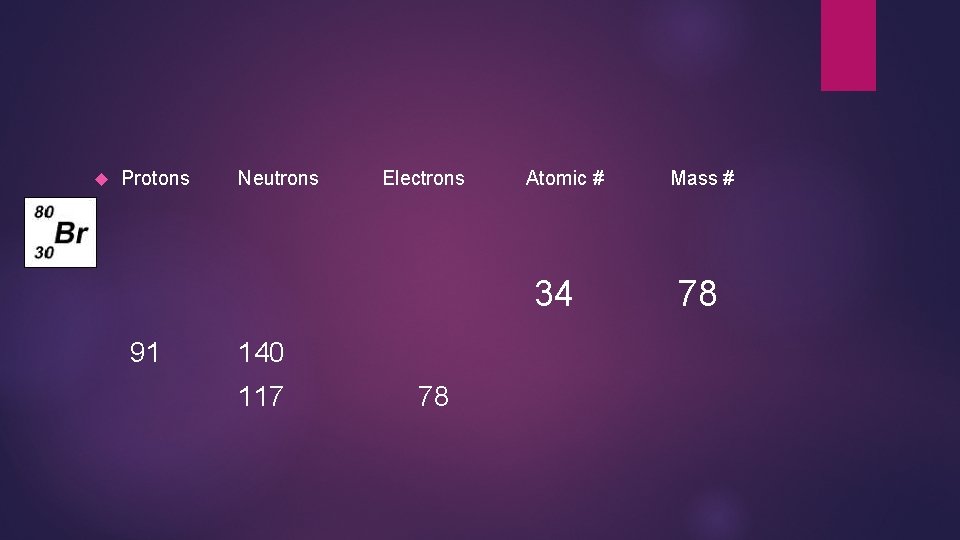
Protons Neutrons Electrons Atomic # 34 91 140 117 78 Mass # 78

Isotopes Dalton was wrong!! All elements are not identical. Atoms of the same element can have different numbers of neutrons, so different mass #. Call them ISOTOPES! 1912 – Frederick Soddy proposed the idea. Won the nobel prize in 1921 for his work.

Naming Isotopes We can put the mass number after the name of the element: p e Carbon-12 H-1 1 1 Protium Carbon-14 H-2 1 1 Uranium-235 Deuterium H-3 1 1 tritium n 0 1 2
Average Atomic Mass Elements occur in nature as mixtures of isotopes. The number on the bottom of the box of each element. This Use is abundance of each isotope in nature. Atomic Mass Unit (amu) – 1/12 of a C-12 atom. Has isotopic purity.
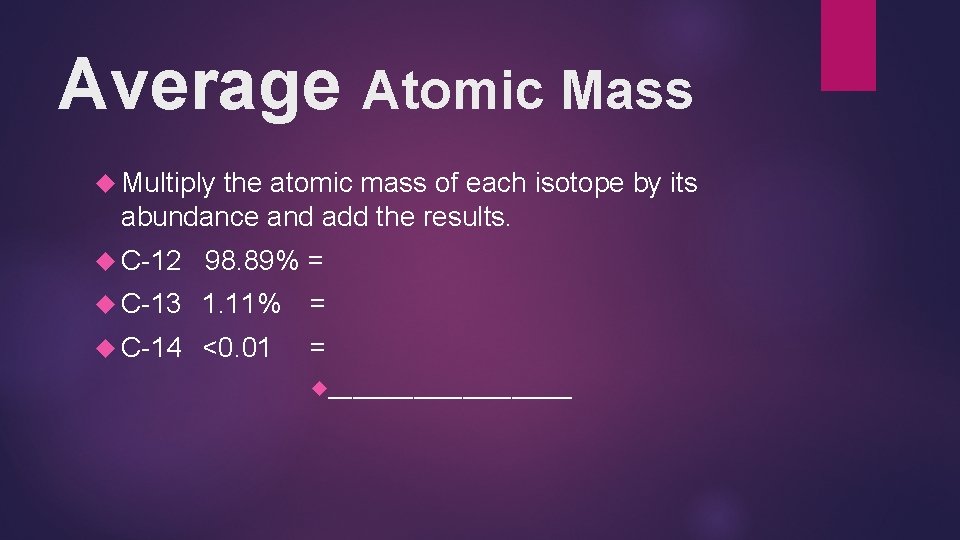
Average Atomic Mass Multiply the atomic mass of each isotope by its abundance and add the results. C-12 98. 89% = C-13 1. 11% = C-14 <0. 01 = __________
- Slides: 10