The Atmosphere Chapter 12 THE AIR AROUND YOU


























- Slides: 27

The Atmosphere Chapter 12

THE AIR AROUND YOU Section 1

Essential Questions 1. What is the composition of Earth’s atmosphere? 2. How is the atmosphere important to living things? 3. What causes smog and acid rain?

§ Weather is the condition of Earth’s atmosphere at a particular time and place. § Earth’s atmosphere is a thin envelope of gases that surrounds the planet and protects it. § It is a thin layer of air forming a protective covering around the planet. (from Glencoe book)

Composition of the Atmosphere § Earth’s atmosphere is made up of nitrogen, oxygen, carbon dioxide, water vapor, and many other gases, as well as particles of liquids and solids.

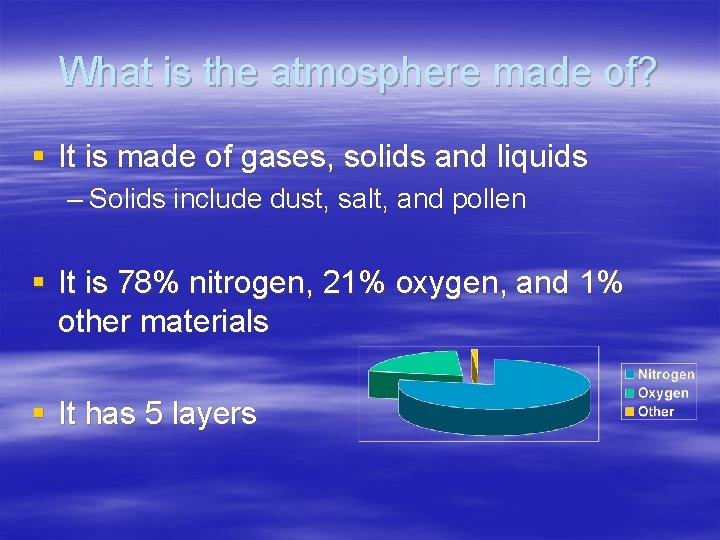
What is the atmosphere made of? § It is made of gases, solids and liquids – Solids include dust, salt, and pollen § It is 78% nitrogen, 21% oxygen, and 1% other materials § It has 5 layers

Nitrogen § § § The most common gas in the atmosphere Makes up more than ¾ of the air we breathe Each molecule consists of two nitrogen atoms § Moves in a cycle from the air to the soil, into living things, and back into the air

Oxygen § Second most abundant gas in the atmosphere § Plants produce oxygen in a process called photosynthesis – they combine water, carbon dioxide, and light § Like nitrogen, oxygen moves through a cycle involving living things § Burning any fuel uses oxygen § Rust is created very slowly by steel reacting to oxygen

Ozone § “supercharged oxygen” § Formed from oxygen with three oxygen atoms in each molecule instead of the usual two § The ozone layer helps protect Earth from ultraviolet radiation given off by the Sun

Carbon Dioxide § Plants use carbon dioxide from the air to make food § Each molecule has one atom of carbon and two atoms of oxygen § Plants and animals give off carbon dioxide § Burning fuels (such as coal and gasoline) releases carbon dioxide into the atmosphere

Other Gases § Argon and carbon dioxide make up most of the 1% of the atmosphere that isn’t nitrogen or oxygen § Trace gases are the other gases in the atmosphere. There are only small amounts of these

Water Vapor § Water in the form of a gas § It is invisible (steam is tiny droplets of water and is NOT water vapor) § Each water molecule contains two atoms of hydrogen and one atom of oxygen § Very important in weather § Clouds form when water vapor condenses to form tiny droplets of water in the air

Particles § Solids and liquids – dust, smoke, salt, and other chemicals § Most are too small to see

Earth’s atmosphere as seen from space

What does the atmosphere do for us? § It traps oxygen close to Earth for us to breathe. § It helps keep the perfect temperature on Earth for plants and us to live. It traps energy from the Sun. § It protects the planet from harmful ultraviolet radiation from the Sun. § It protects us from space objects striking Earth (such as meteoroids – space rocks) § Because the atmosphere is constantly changing, the air can be considered a renewable resource

Asteroid grazing Earth’s atmosphere

What would life be like on Earth without the atmosphere? § During the day temperatures would rise very high and probably be too hot for life. § During the night temperatures would drop to very cold levels.

Air Quality § The air we breathe into our bodies may be filled with harmful chemicals and particles § Pollutants are harmful substances in the air, water, or soil § Air pollution can affect the health of humans and other living things

Sources of Pollution § Natural processes can add particles to the atmopshere – Forest fires, soil erosion, dust storms all add smoke and dust to the air – Wind carries molds and pollens – Erupting volcanoes spew out dust, ash, and poisonous gases – Burning fossil fuels adds carbon monoxide, nitrogen oxides, and sulfur oxides to the air

Smog & Acid Rain § § § Caused by the burning of fossil fuels Smog = smoke + fog; a type of air pollution Photochemical smog – formed when sunlight acts upon hydrocarbons and nitrogen oxides. It is a brown haze in the sky. § Can be harmful to plants and can irritate humans

Acid Rain § Rain that contains more acid than normal § Caused when water vapor mixes with harmful nitrogen oxides and sulfur oxides in the air § Can be strong enough to damage surfaces of buildings and statues § Harmful to lakes and ponds

Improving Air Quality § The US has many laws to protect and clean up the air § The Environmental Protection Agency (EPA) monitors air pollutants in the US § Air quality has improved because of improvements to automobiles and power plants § More cars and more power plants are needed now, so air quality still needs to improve

Solutions for Improving Air Quality § Use public transportation as much as possible § Stricter regulations might be needed § Could this cost too much?

Review Quiz 1. The most abundant gas in the atmosphere is a. b. c. d. Ozone Water vapor Oxygen Nitrogen

Quiz continued 2. ______ is the condition of Earth’s atmosphere at a particular time and place. a. b. c. d. Climate Temperature Weather Ozone

Quiz continued 3. True or False: Earth’s atmosphere makes conditions on Earth suitable for living things. 4. Smog and acid rain can be caused from burning a. b. c. d. Coal Petroleum oil Natural gas All of the above

Essential Questions Answered 1. What is the composition of Earth’s atmosphere? 78% nitrogen, 21% oxygen, 1% other 2. How is the atmosphere important to living things? It holds oxygen, keeps Earth warm, and protects us from UV rays and meteors. 3. What causes smog and acid rain? Pollution from burning fossil fuels