The Atmosphere as a Chemical Reactor Radiation energy






![Production of OH Radical Solve for [O(1 D)] Substitute into the production of OH Production of OH Radical Solve for [O(1 D)] Substitute into the production of OH](https://slidetodoc.com/presentation_image_h2/a4922a8645fb69eb24e44271a8eb9453/image-18.jpg)

- Slides: 27

The Atmosphere as a Chemical Reactor Radiation (energy) Inputs Outputs Chemistry Biogeochemical Cycling

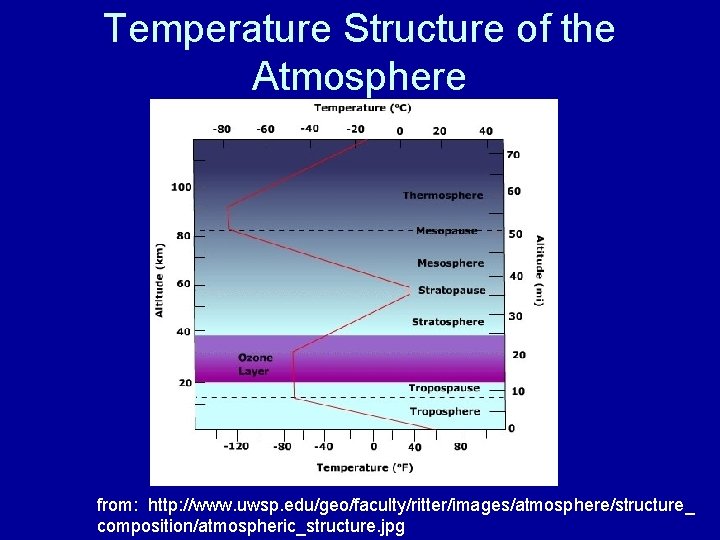
Temperature Structure of the Atmosphere from: http: //www. uwsp. edu/geo/faculty/ritter/images/atmosphere/structure_ composition/atmospheric_structure. jpg

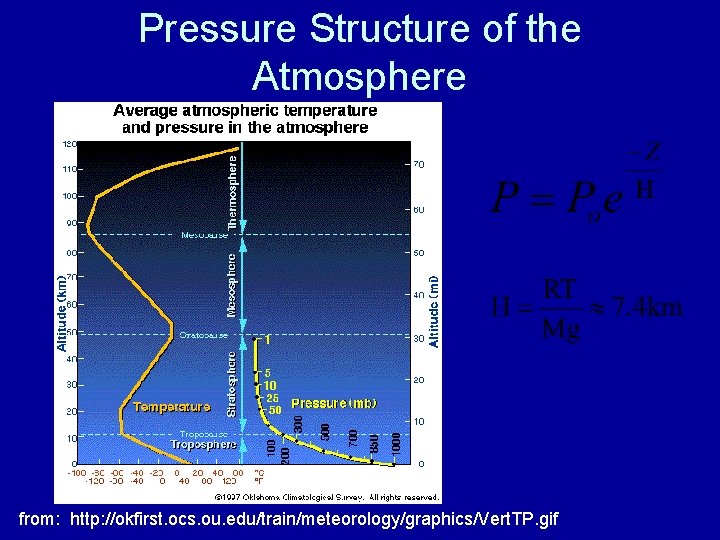
Pressure Structure of the Atmosphere from: http: //okfirst. ocs. ou. edu/train/meteorology/graphics/Vert. TP. gif

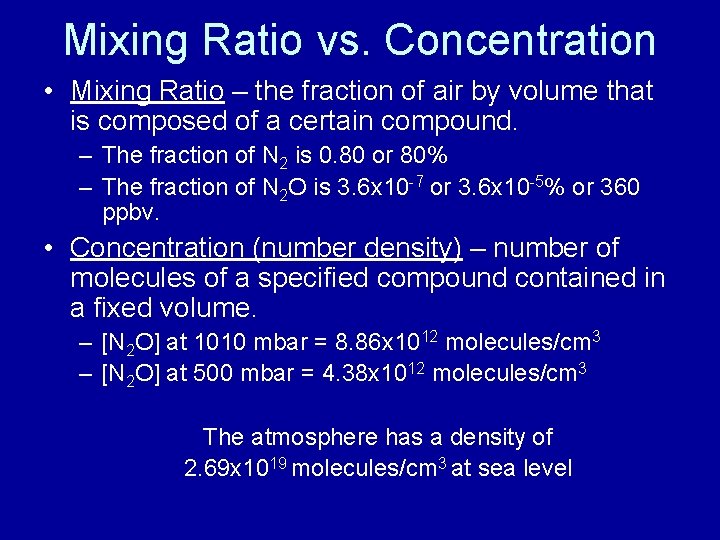
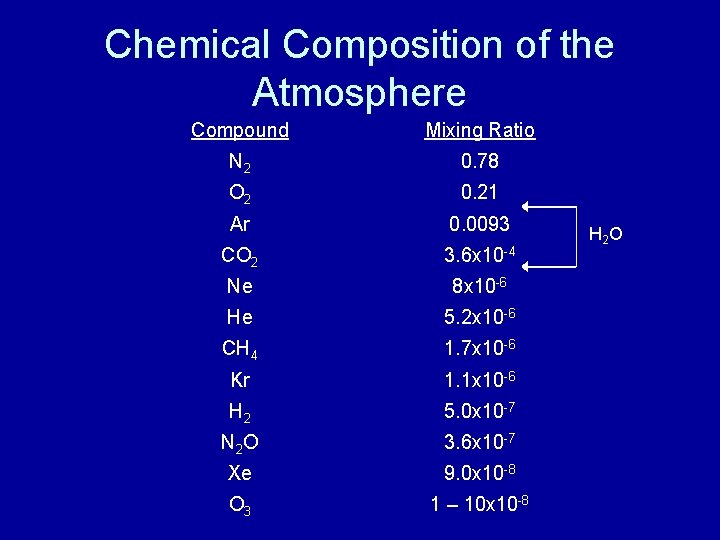
Mixing Ratio vs. Concentration • Mixing Ratio – the fraction of air by volume that is composed of a certain compound. – The fraction of N 2 is 0. 80 or 80% – The fraction of N 2 O is 3. 6 x 10 -7 or 3. 6 x 10 -5% or 360 ppbv. • Concentration (number density) – number of molecules of a specified compound contained in a fixed volume. – [N 2 O] at 1010 mbar = 8. 86 x 1012 molecules/cm 3 – [N 2 O] at 500 mbar = 4. 38 x 1012 molecules/cm 3 The atmosphere has a density of 2. 69 x 1019 molecules/cm 3 at sea level

Chemical Composition of the Atmosphere Compound Mixing Ratio N 2 0. 78 O 2 0. 21 Ar 0. 0093 CO 2 3. 6 x 10 -4 Ne 8 x 10 -6 He 5. 2 x 10 -6 CH 4 1. 7 x 10 -6 Kr 1. 1 x 10 -6 H 2 5. 0 x 10 -7 N 2 O 3. 6 x 10 -7 Xe 9. 0 x 10 -8 O 3 1 – 10 x 10 -8 H 2 O

Vertical Mixing of the Troposphere • Boundary Layer – hours to days • Free Troposphere – days to weeks • Tropopause - years

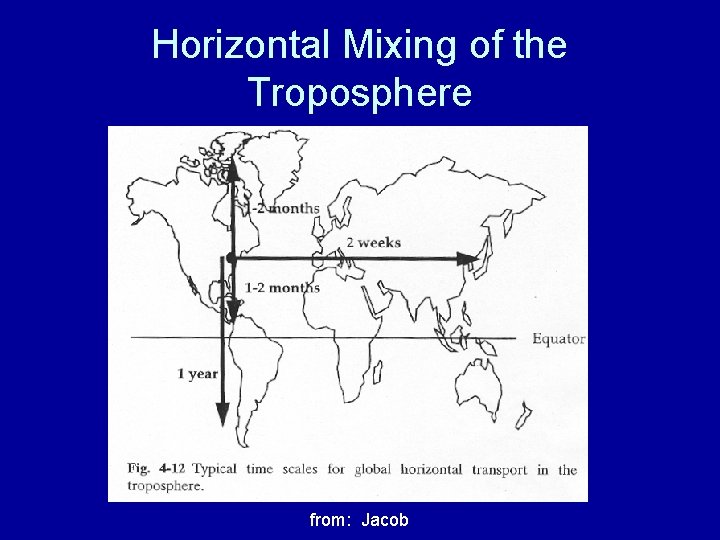
Horizontal Mixing of the Troposphere from: Jacob

Horizontal Mixing of the Troposphere

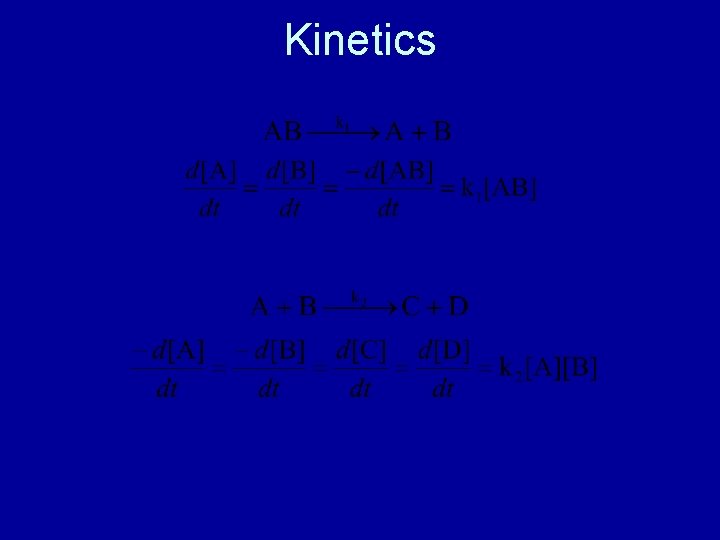
Kinetics

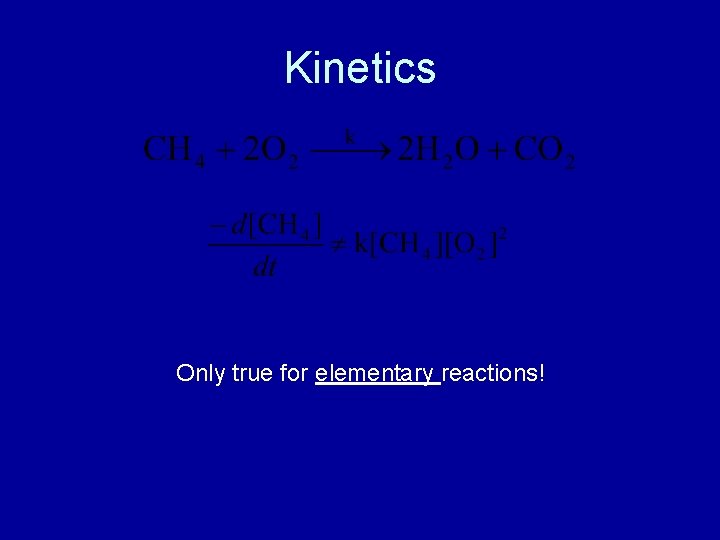
Kinetics Only true for elementary reactions!

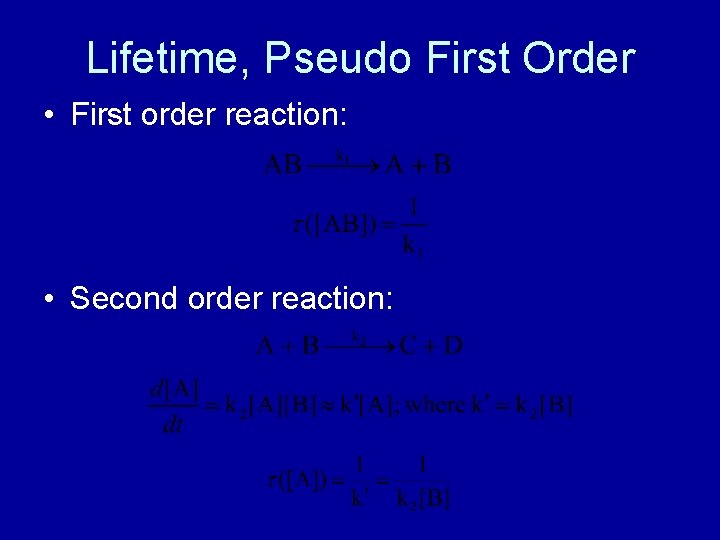
Lifetime, Pseudo First Order • First order reaction: • Second order reaction:

Lifetime • Some compounds have multiple sinks • Methane chemical loss in troposphere by OH ≈ 12 yrs physical deposition to the soil ≈ 100 yrs chemical loss in stratosphere ≈ 40 yrs Overall lifetime:

Rate Constants

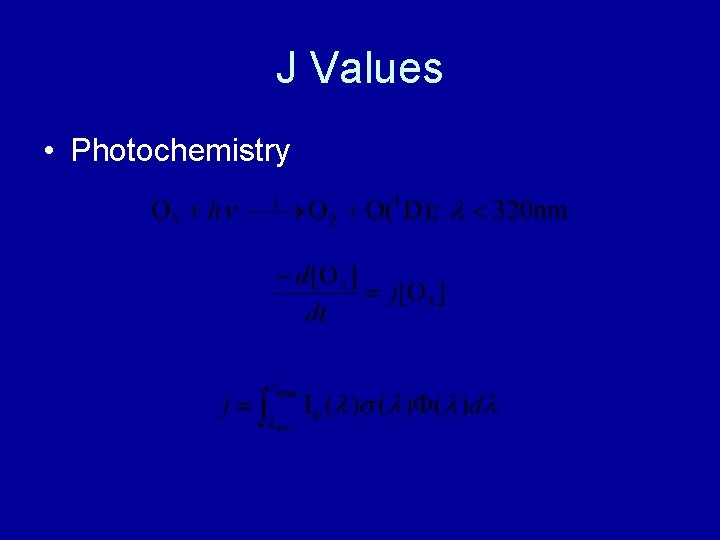
J Values • Photochemistry

Radical Chemistry • Radicals do the work of the chemistry in the atmosphere. • Produced by photochemical reactions and reactions by other radicals • O(3 P) is a bi-radical • O(1 D) is not a radical, but it is very reactive

The OH Radical

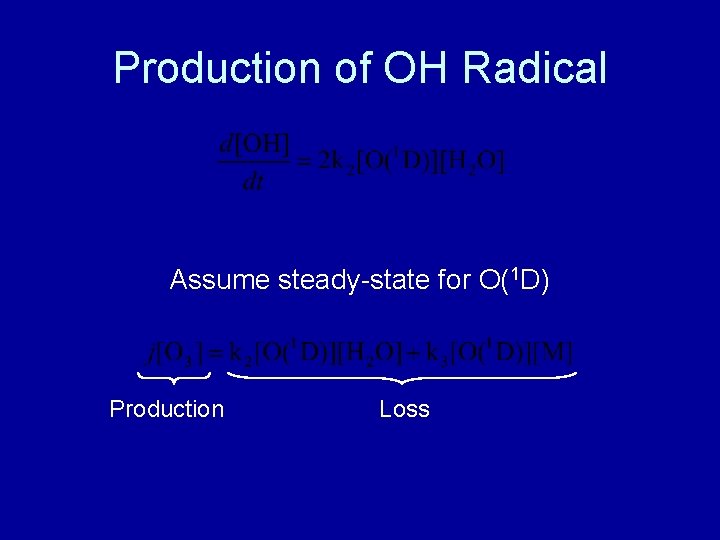
Production of OH Radical Assume steady-state for O(1 D) Production Loss
![Production of OH Radical Solve for O1 D Substitute into the production of OH Production of OH Radical Solve for [O(1 D)] Substitute into the production of OH](https://slidetodoc.com/presentation_image_h2/a4922a8645fb69eb24e44271a8eb9453/image-18.jpg)
Production of OH Radical Solve for [O(1 D)] Substitute into the production of OH

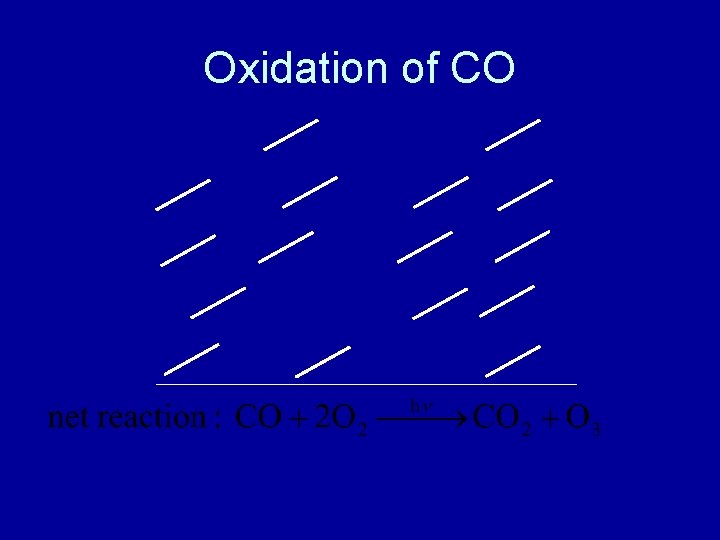
Oxidation of CO

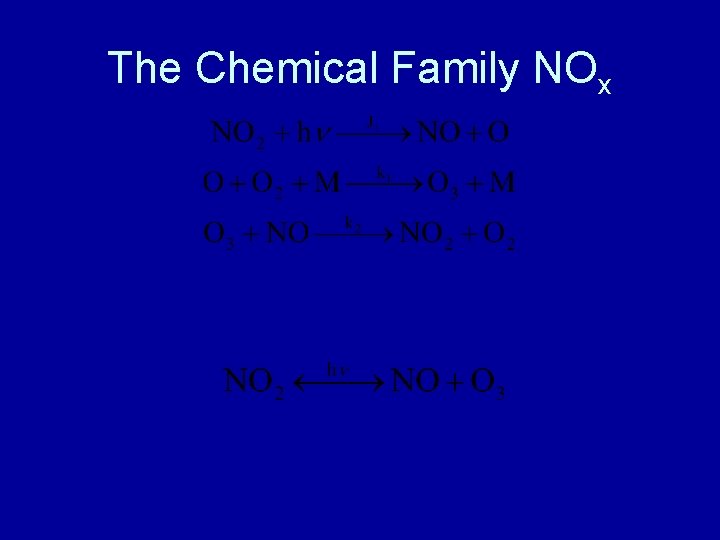
The Chemical Family NOx

The Chemical Family NOx

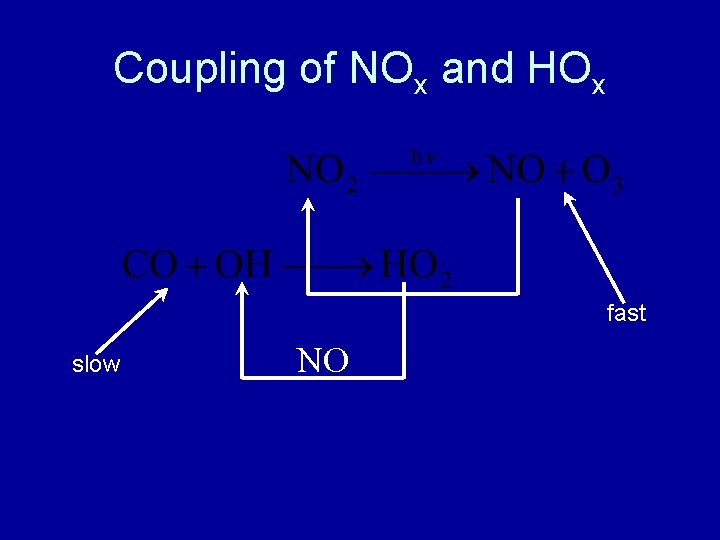
Coupling of NOx and HOx fast slow NO

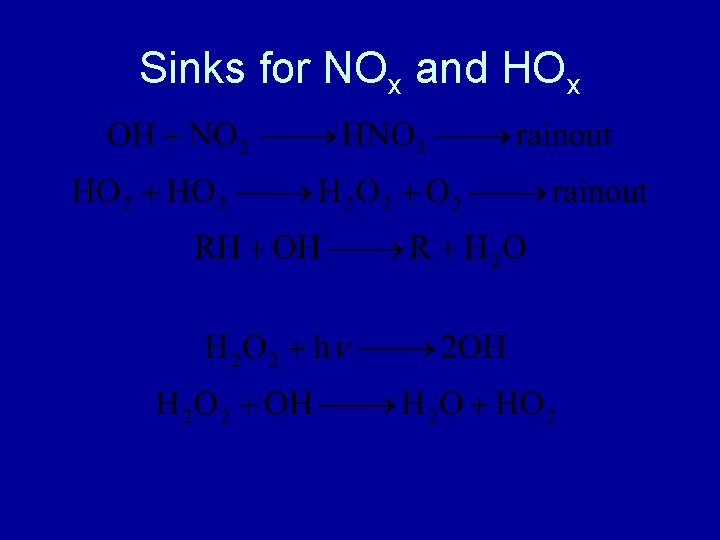
Sinks for NOx and HOx

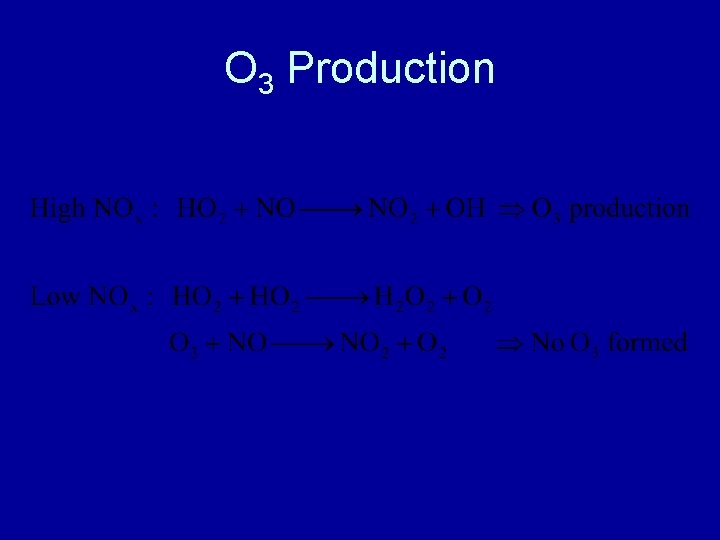
O 3 Production

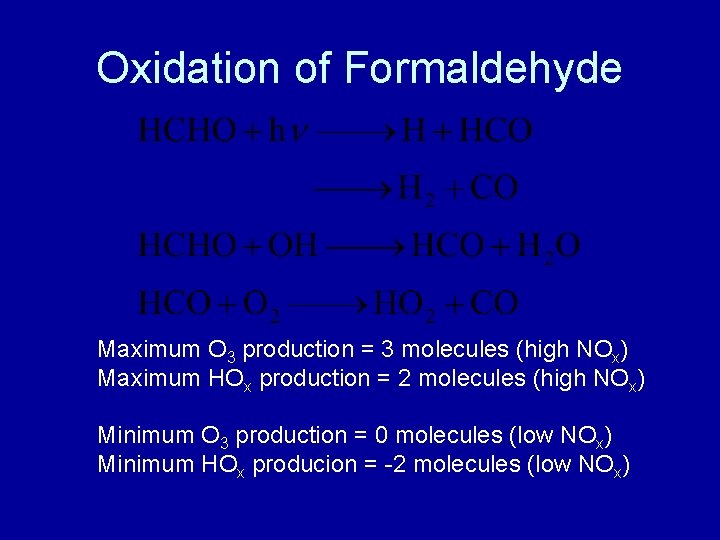
Oxidation of Formaldehyde Maximum O 3 production = 3 molecules (high NOx) Maximum HOx production = 2 molecules (high NOx) Minimum O 3 production = 0 molecules (low NOx) Minimum HOx producion = -2 molecules (low NOx)

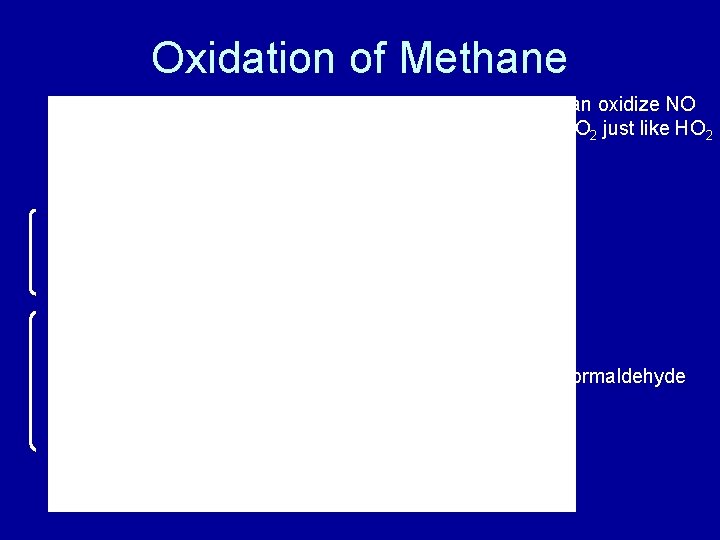
Oxidation of Methane Can oxidize NO to NO 2 just like HO 2 formaldehyde

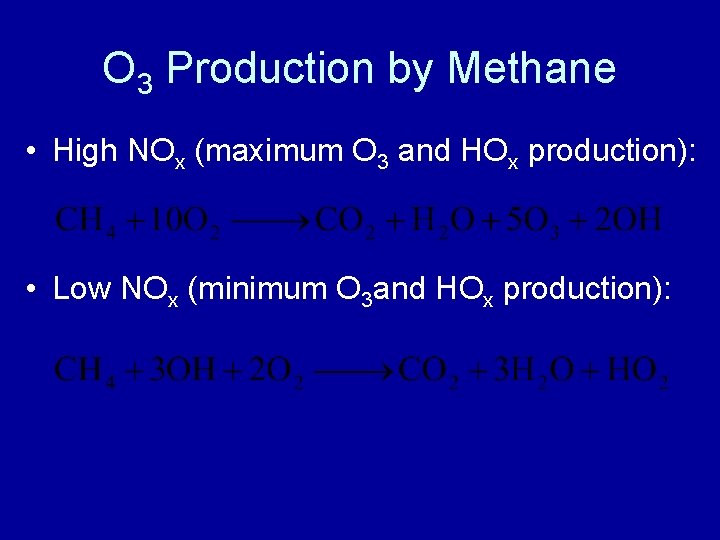
O 3 Production by Methane • High NOx (maximum O 3 and HOx production): • Low NOx (minimum O 3 and HOx production):