The Atmosphere Air l Made up of 78






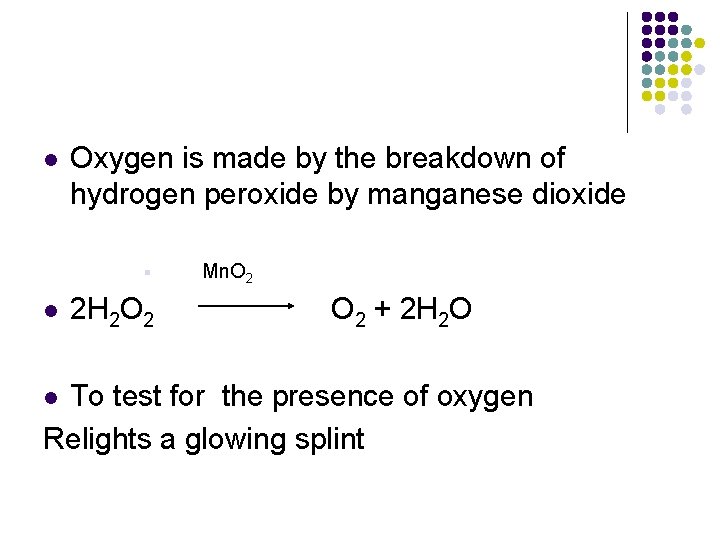




- Slides: 17

The Atmosphere

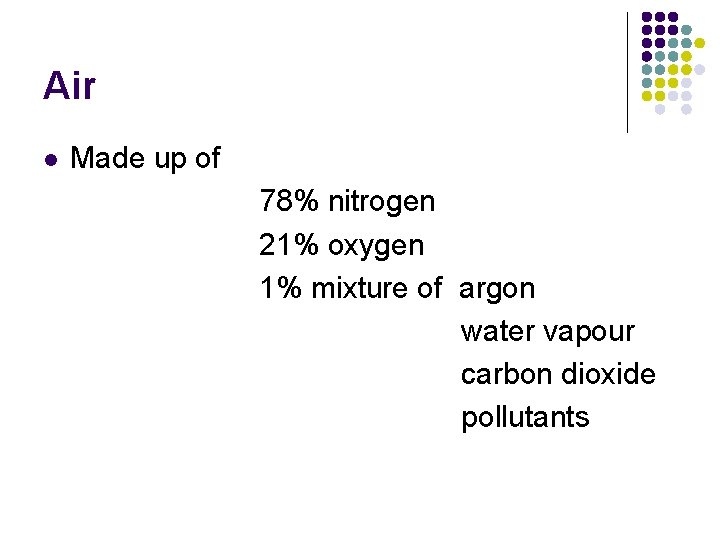
Air l Made up of 78% nitrogen 21% oxygen 1% mixture of argon water vapour carbon dioxide pollutants

l Humidity is a measure of the amount of water vapour in the air

To show 1/5 th of air is oxygen Result The steel wool rusts using the oxygen in the air in the cylinder Water rises up the cylinder to replace the oxygen used up The water rises to approx 1/5 of the height of the cylinder l

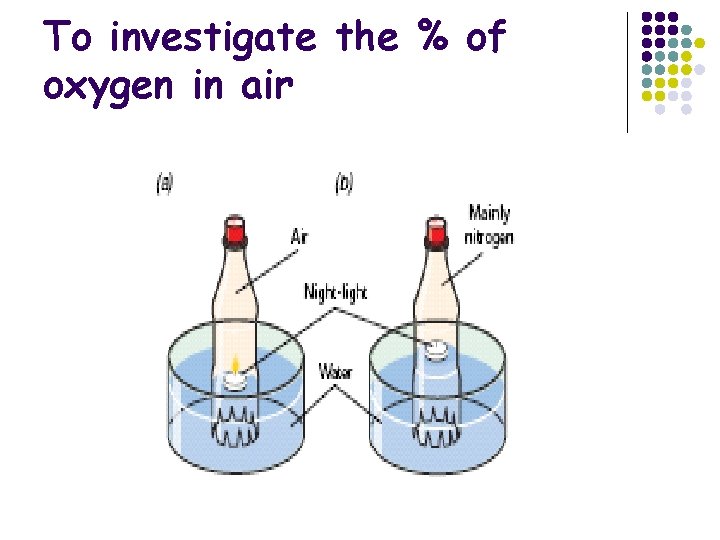
To investigate the % of oxygen in air

To investigate the % of oxygen in air Calculation Volume of air in bottle at beginning Volume of gas in bottle after night light burnt Volume of oxygen used up Conclusion Oxygen occupies approximately one fifth of air

Carbon dioxide in the air l l Chemical used --- limewater Result---- turns lime water milky WATER IN THE AIR l Chemical used--- cobalt chloride paper l Result--- turn from blue to pink in the presence of water

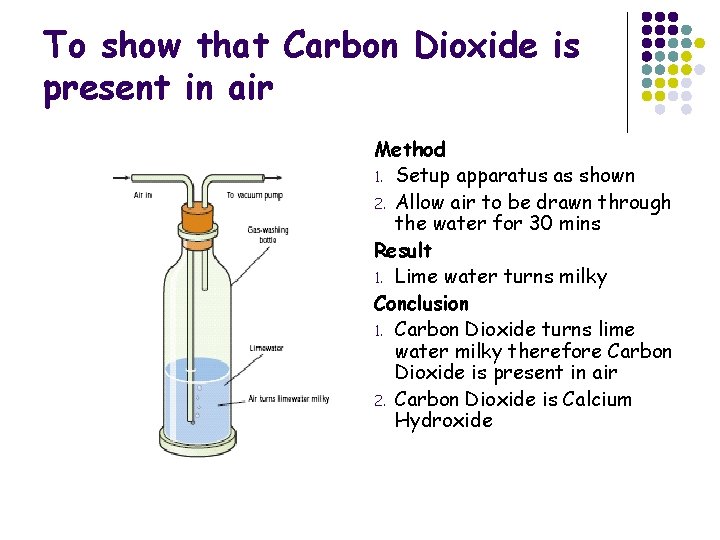
To show that Carbon Dioxide is present in air Method 1. Setup apparatus as shown 2. Allow air to be drawn through the water for 30 mins Result 1. Lime water turns milky Conclusion 1. Carbon Dioxide turns lime water milky therefore Carbon Dioxide is present in air 2. Carbon Dioxide is Calcium Hydroxide

Making oxygen l A catalyst is used in the porcess of making oxygen in the lab l CATALYSTS--- are substances that alter the rate of chemical reactions but are not used up themselves in the reaction

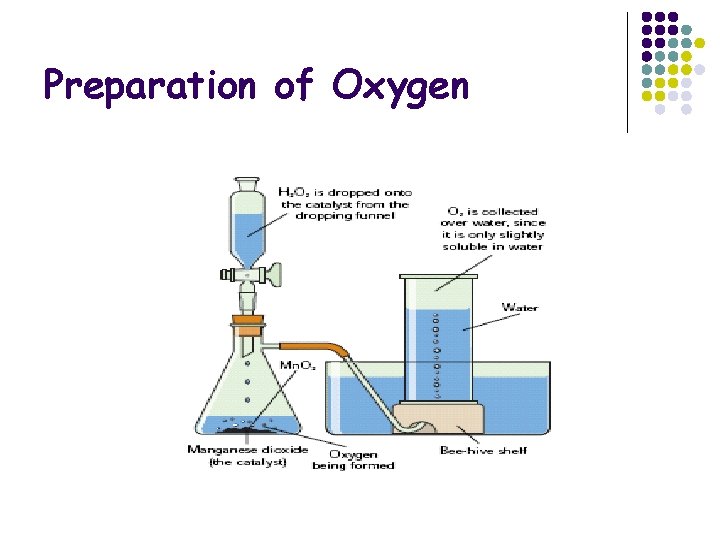
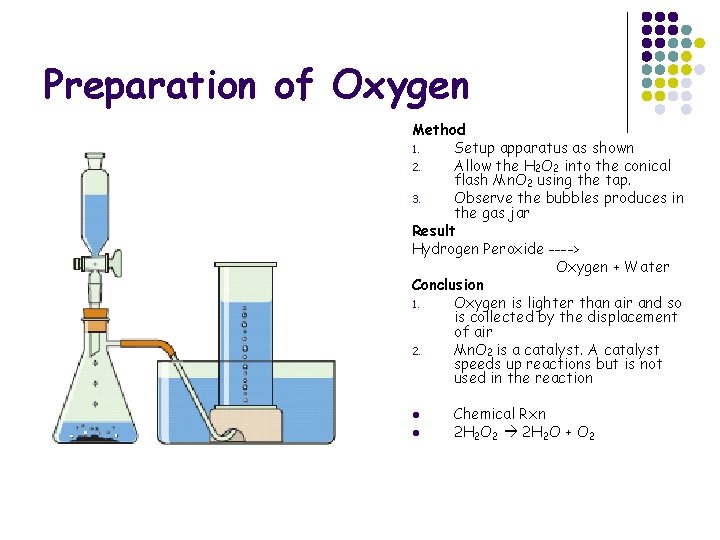
Preparation of Oxygen
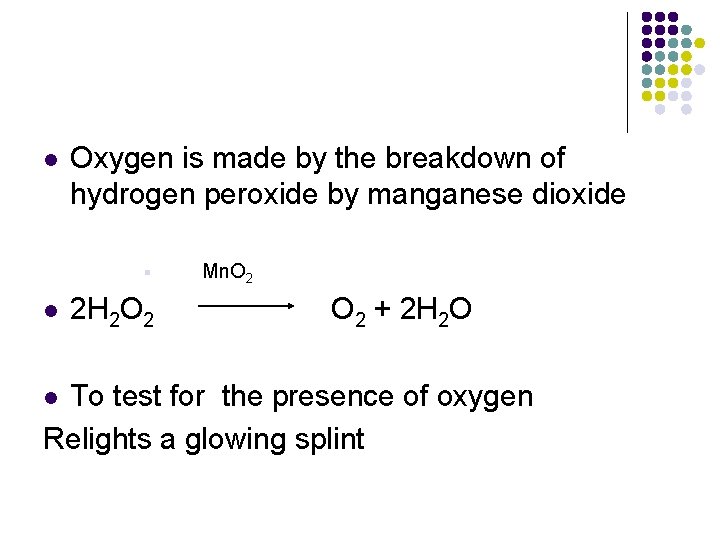
l Oxygen is made by the breakdown of hydrogen peroxide by manganese dioxide § l 2 H 2 O 2 Mn. O 2 + 2 H 2 O To test for the presence of oxygen Relights a glowing splint l

Preparation of Oxygen Method 1. Setup apparatus as shown 2. Allow the H 2 O 2 into the conical flash Mn. O 2 using the tap. 3. Observe the bubbles produces in the gas jar Result Hydrogen Peroxide ----> Oxygen + Water Conclusion 1. Oxygen is lighter than air and so is collected by the displacement of air 2. Mn. O 2 is a catalyst. A catalyst speeds up reactions but is not used in the reaction l l Chemical Rxn 2 H 2 O 2 2 H 2 O + O 2

To examine the properties of Oxygen

To prepare carbon dioxide l By reacting dilute hydrochloric acid( HCl) with calcium carbonate( Ca. CO 3) l Ca. CO 3 + 2 HCl l to test for carbon dioxide Place a lighted splint in a jar of carbon dioxide--- the splint goes out showing CO 2 is present as CO 2 does not support combustion If you bubble CO 2 into limewater the limewater turns milky showing CO 2 is present l l Ca. Cl 2 + H 2 O + CO 2

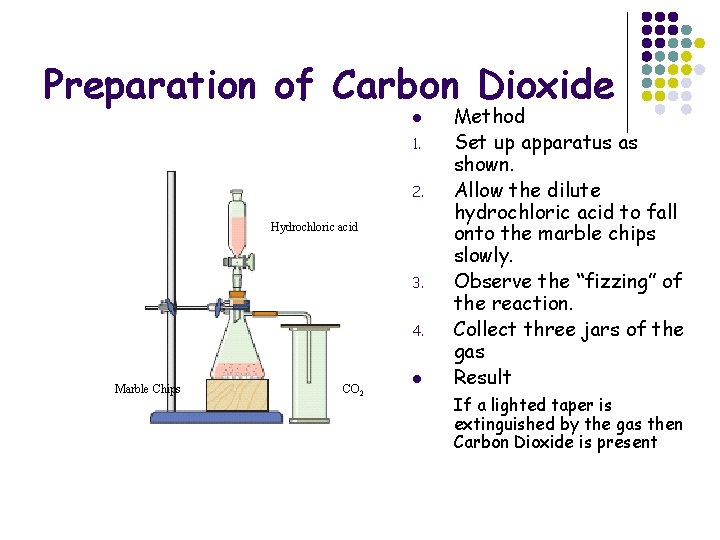
Preparation of Carbon Dioxide l 1. 2. Hydrochloric acid 3. 4. Marble Chips CO 2 l Method Set up apparatus as shown. Allow the dilute hydrochloric acid to fall onto the marble chips slowly. Observe the “fizzing” of the reaction. Collect three jars of the gas Result If a lighted taper is extinguished by the gas then Carbon Dioxide is present


Main Uses of Carbon Dioxide CO 2 l Fizzy Drinks l l Fire Extinguishers l l CO 2 is denser than air and does not support combustion Refrigeration l l All fizzy drinks contain CO 2 under pressure can be converted into a solid: Dry Ice. It is much colder than ice – 78 o. C Dry Ice- Special Effects on Stage l Lumps of dry Ice are put into warm water and wet CO 2 carries clouds of water vapour with it. Mist Effect
 Air higroskopis adalah
Air higroskopis adalah Edible aquifer lab activity answers
Edible aquifer lab activity answers Dessert made by adding air to a flavored base
Dessert made by adding air to a flavored base Sectional air map made of plastic
Sectional air map made of plastic What is air made of
What is air made of Plokmijnuhbygvtfcrdxeszwaq games
Plokmijnuhbygvtfcrdxeszwaq games Tư thế ngồi viết
Tư thế ngồi viết Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Thẻ vin
Thẻ vin V. c c
V. c c Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Slidetodoc
Slidetodoc