The Arrhenius Equation Collision Theory A bimolecular reaction
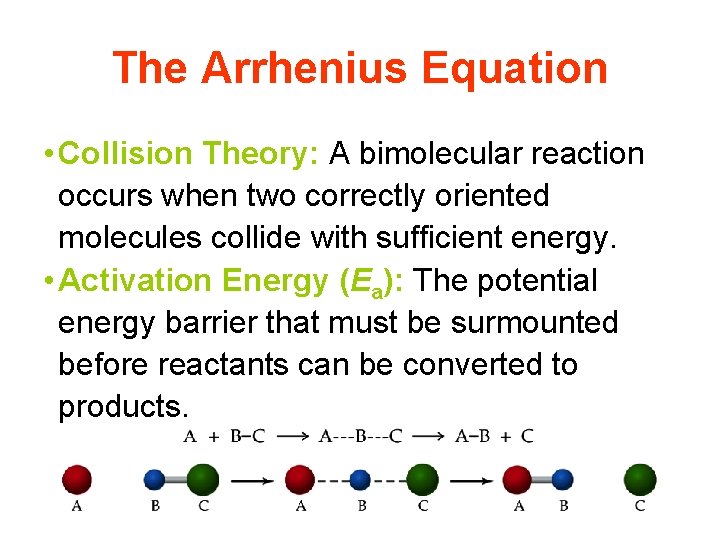
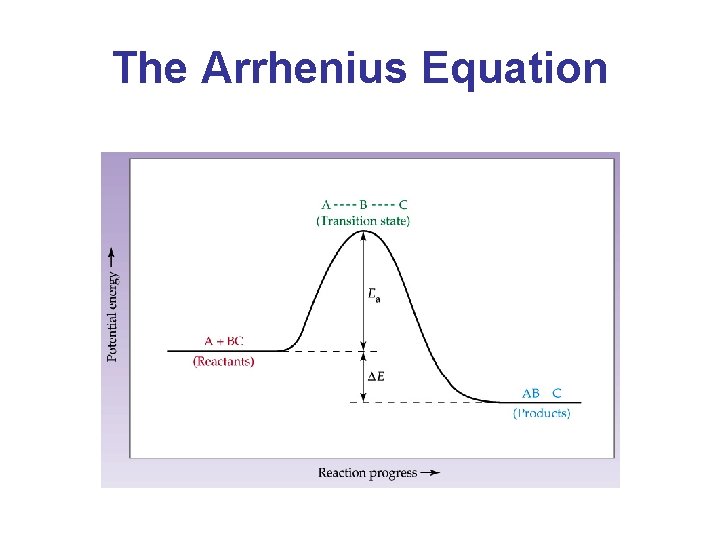
The Arrhenius Equation • Collision Theory: A bimolecular reaction occurs when two correctly oriented molecules collide with sufficient energy. • Activation Energy (Ea): The potential energy barrier that must be surmounted before reactants can be converted to products.
The Arrhenius Equation
The Arrhenius Equation
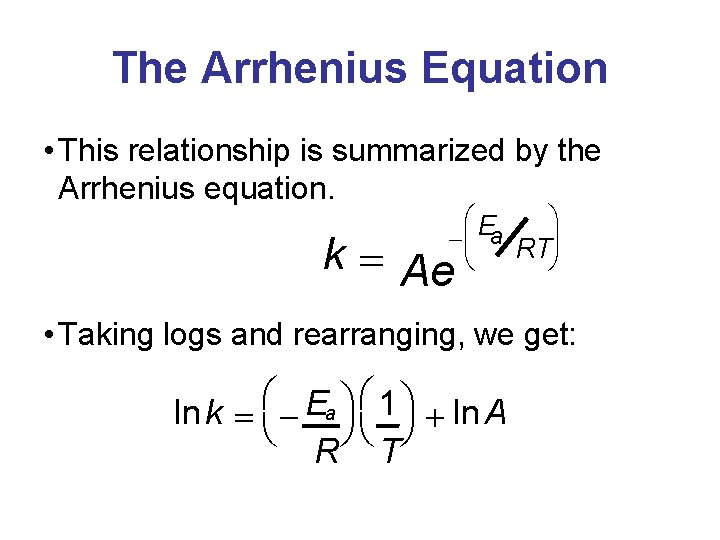
The Arrhenius Equation • This relationship is summarized by the Arrhenius equation. æE ö a -ç ÷ RT è ø k = Ae • Taking logs and rearranging, we get: æ æ ö E ln k = - a 1 ö + ln A è øè ø R T

The Arrhenius Equation Temp k (°C) (M-1 s-1) 283 356 393 3. 52 e-7 3. 02 e 5 2. 19 e-4 427 508 1. 16 e-3 3. 95 e-2
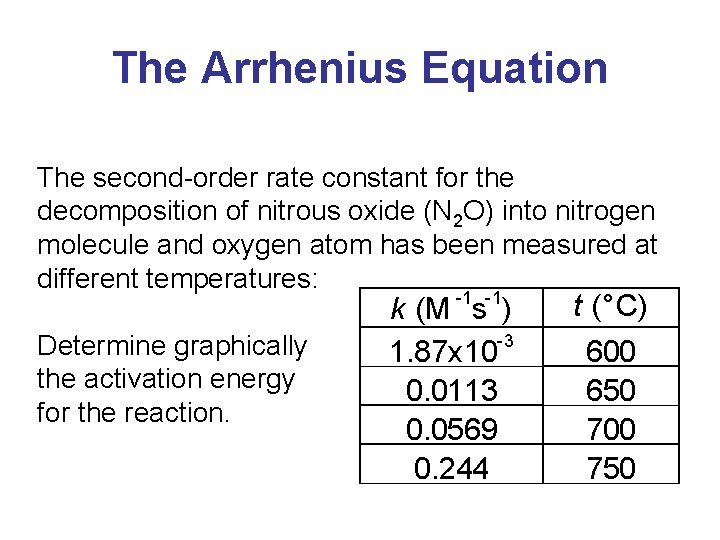
The Arrhenius Equation The second-order rate constant for the decomposition of of nitrous oxide (N (N 22 O) O) into nitrogen molecule and oxygen atom has been measured at at different temperatures: -1 -1 Determine graphically the activation energy for the reaction. kk (M (M ss )) -3 -3 1. 87 x 10 0. 0113 0. 0569 0. 244 tt (°C) 600 650 700 750

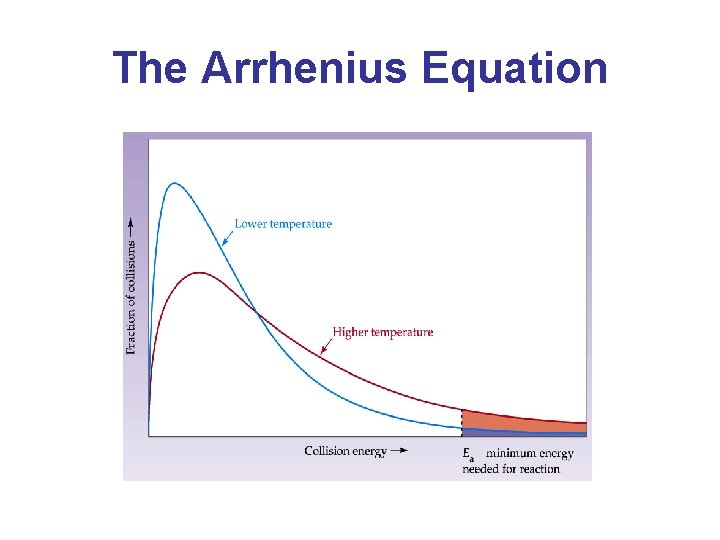
The Arrhenius Equation • A simpler way to use this is by comparing the rate constant at just two temperatures: • If the rate of a reaction doubles by increasing the temperature by 10°C from 298. 2 K to 308. 2 K, what is the activation energy of the reaction?
- Slides: 7