The Anthropogenic Greenhouse Effect Anthropogenic Greenhouse Effect The








- Slides: 14

The Anthropogenic Greenhouse Effect

Anthropogenic Greenhouse Effect “The enhancement of the natural greenhouse effect due to human activity. ” The Problem: • The increase in greenhouse gas levels may trap more IR in the atmosphere, increasing the average global temperature.

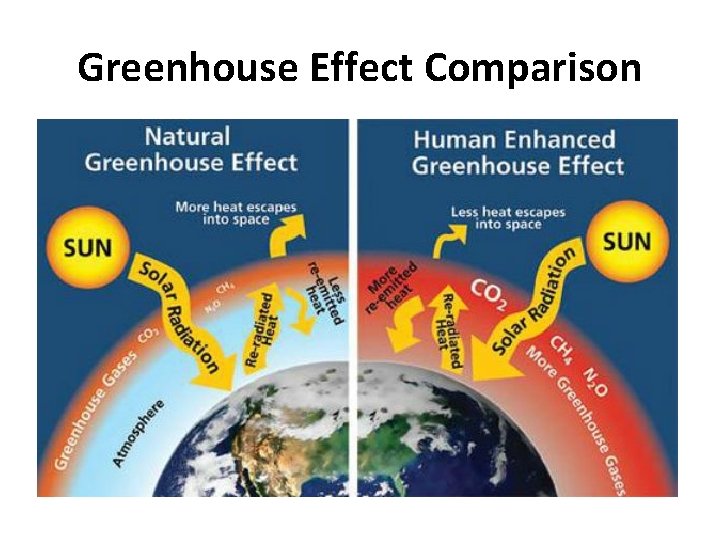
Greenhouse Effect Comparison

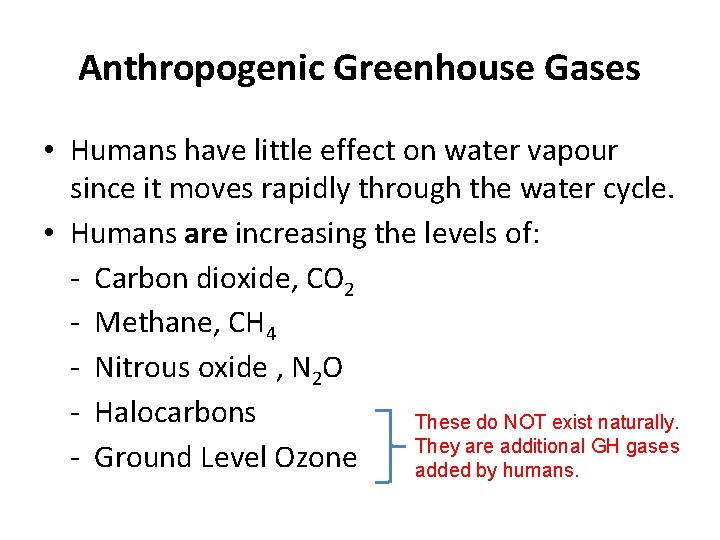
Anthropogenic Greenhouse Gases • Humans have little effect on water vapour since it moves rapidly through the water cycle. • Humans are increasing the levels of: - Carbon dioxide, CO 2 - Methane, CH 4 - Nitrous oxide , N 2 O - Halocarbons These do NOT exist naturally. They are additional GH gases - Ground Level Ozone added by humans.

Carbon Dioxide Sources: • Combustion of fossil fuels. – Transportation, heating, generating electricity, etc. • Burning forests to clear land. • Cement making.

Methane Sources: • Coal and natural gas processing. • Agriculture (manure). • Landfill sites (garbage).

Nitrous Oxide Sources: • Combustion of fossil fuels. • Chemical fertilizers. • Manure and sewage.

Halocarbons • Synthetic compounds containing carbon and halogen elements (F, Cl, Br). • Used as cleaners, solvents, and coolants. • Includes CFCs (chlorofluorocarbons).

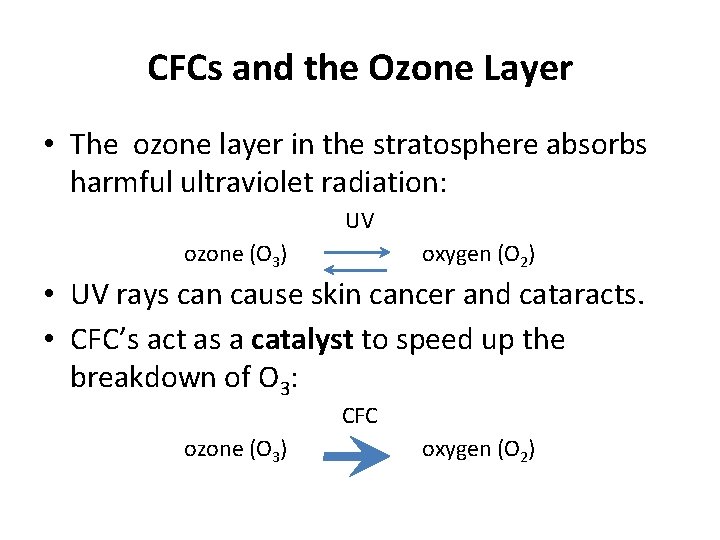
CFCs and the Ozone Layer • The ozone layer in the stratosphere absorbs harmful ultraviolet radiation: UV ozone (O 3) oxygen (O 2) • UV rays can cause skin cancer and cataracts. • CFC’s act as a catalyst to speed up the breakdown of O 3: CFC ozone (O 3) oxygen (O 2)

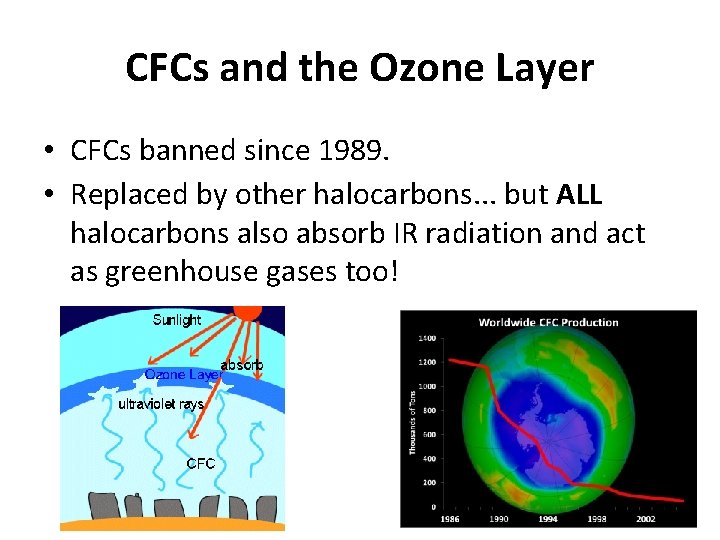
CFCs and the Ozone Layer • CFCs banned since 1989. • Replaced by other halocarbons. . . but ALL halocarbons also absorb IR radiation and act as greenhouse gases too!

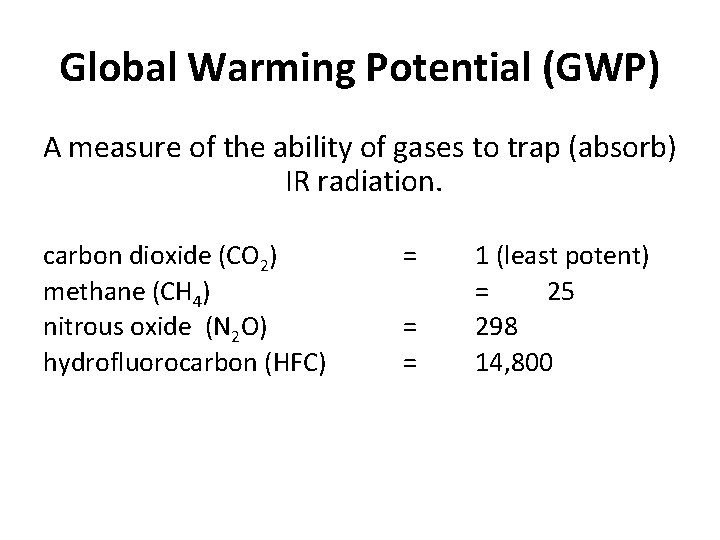
Global Warming Potential (GWP) A measure of the ability of gases to trap (absorb) IR radiation. carbon dioxide (CO 2) methane (CH 4) nitrous oxide (N 2 O) hydrofluorocarbon (HFC) = = = 1 (least potent) = 25 298 14, 800

Why Focus on Carbon? • CO 2 has a low GWP but it is the most abundant greenhouse gas. • It is also a greenhouse gas that humans directly impact on a large scale. • Fossil fuel use has increased exponentially since the 1800 s. • Fossil fuel extraction and combustion also releases N 2 O and CH 4.

Ground Level Ozone • A major component of smog. • Produced by photochemical reactions between N 2 O and volatile organic compounds in the presence of sunlight. 13

Ground Level Ozone • Higher levels occur from May to September, between noon and early evening. • Very harmful to environment: damages plants and severe respiratory health problems in humans and other animals. 14
 Gmail.com
Gmail.com Is the greenhouse effect good or bad
Is the greenhouse effect good or bad Why is the greenhouse effect important
Why is the greenhouse effect important Greenhouse effect powerpoint
Greenhouse effect powerpoint Enhanced greenhouse effect
Enhanced greenhouse effect Greenhouse effect
Greenhouse effect Greenhouse effect
Greenhouse effect Greenhouse effect gif
Greenhouse effect gif Whats a green house
Whats a green house Causes of greenhouse effect
Causes of greenhouse effect Slidetodoc
Slidetodoc Human activities that enhanced greenhouse effect
Human activities that enhanced greenhouse effect Greenhouse effect on plants
Greenhouse effect on plants Greenhouse effect
Greenhouse effect Biodiversity definition ap human geography
Biodiversity definition ap human geography