The Advanced Chemical Engineering Thermodynamics The thermodynamics properties

![Property relations ¡ The residual enthalpy, H /RT= - T [ (GR/RT)/ T]P, The Property relations ¡ The residual enthalpy, H /RT= - T [ (GR/RT)/ T]P, The](https://slidetodoc.com/presentation_image_h/bef3b3b70aa11f7f215f64fbdf8f8c1f/image-6.jpg)



- Slides: 28

The Advanced Chemical Engineering Thermodynamics The thermodynamics properties of fluids (II) Q&A_-10 - 11/17/2005(10) Ji-Sheng Chang

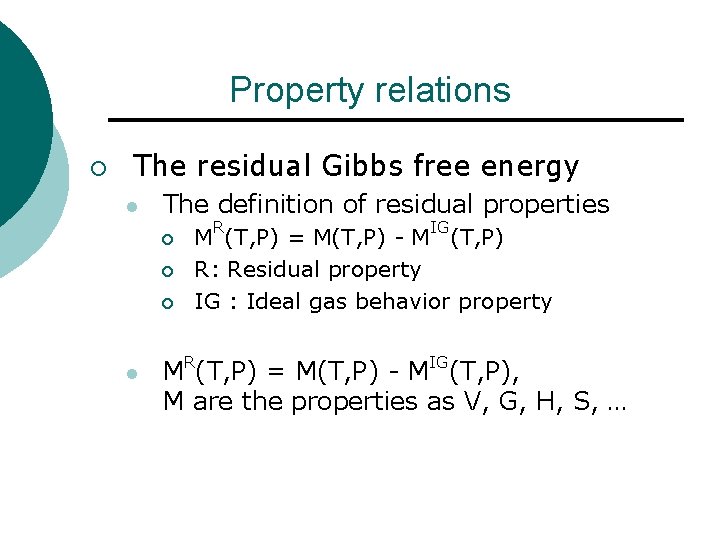
Property relations ¡ The residual Gibbs free energy l The definition of residual properties ¡ ¡ ¡ l R IG M (T, P) = M(T, P) - M (T, P) R: Residual property IG : Ideal gas behavior property R IG M (T, P) = M(T, P) - M (T, P), M are the properties as V, G, H, S, …

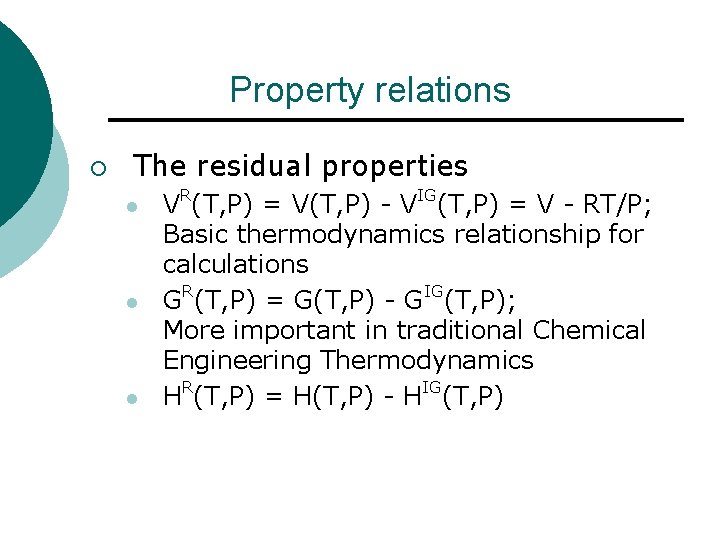
Property relations ¡ The residual properties l l l VR(T, P) = V(T, P) - VIG(T, P) = V - RT/P; Basic thermodynamics relationship for calculations GR(T, P) = G(T, P) - GIG(T, P); More important in traditional Chemical Engineering Thermodynamics HR(T, P) = H(T, P) - HIG(T, P)

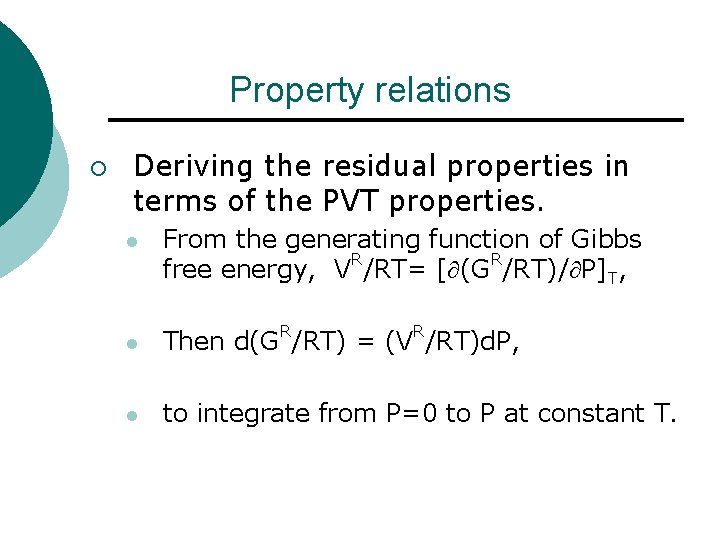
Property relations ¡ Deriving the residual properties in terms of the PVT properties. l From the generating function of Gibbs free energy, VR/RT= [ (GR/RT)/ P]T, l Then d(GR/RT) = (VR/RT)d. P, l to integrate from P=0 to P at constant T.

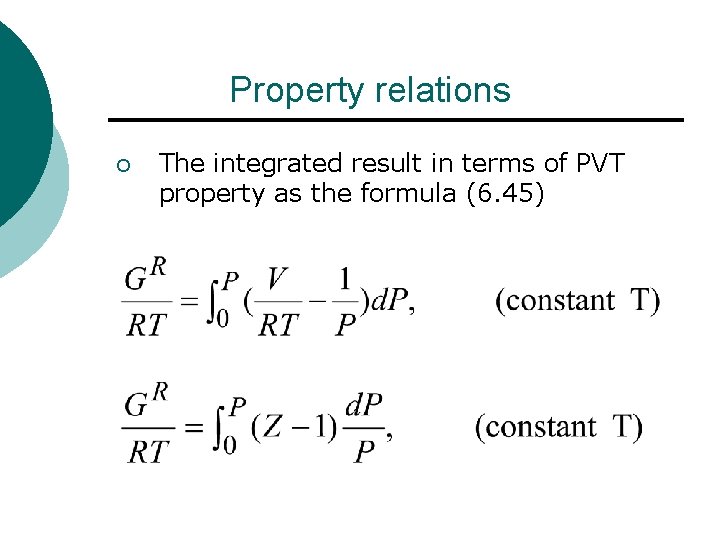
Property relations ¡ The integrated result in terms of PVT property as the formula (6. 45)
![Property relations The residual enthalpy H RT T GRRT TP The Property relations ¡ The residual enthalpy, H /RT= - T [ (GR/RT)/ T]P, The](https://slidetodoc.com/presentation_image_h/bef3b3b70aa11f7f215f64fbdf8f8c1f/image-6.jpg)
Property relations ¡ The residual enthalpy, H /RT= - T [ (GR/RT)/ T]P, The integrated result in terms of PVT property as the formula (6. 46) R

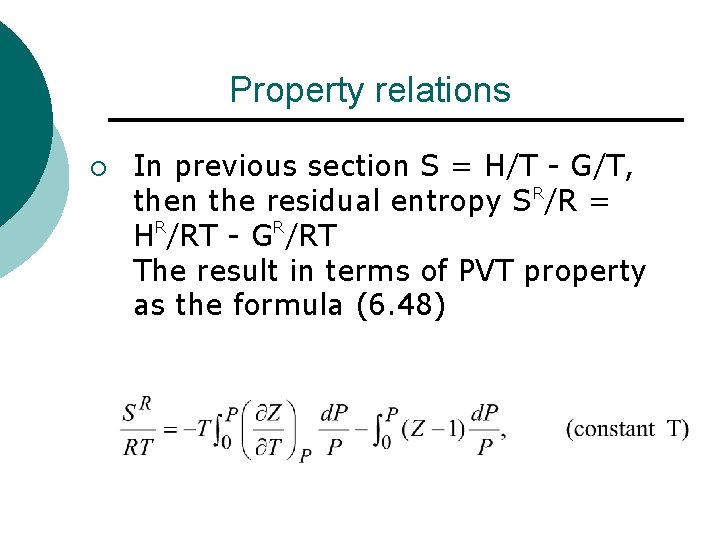
Property relations ¡ In previous section S = H/T - G/T, then the residual entropy SR/R = HR/RT - GR/RT The result in terms of PVT property as the formula (6. 48)

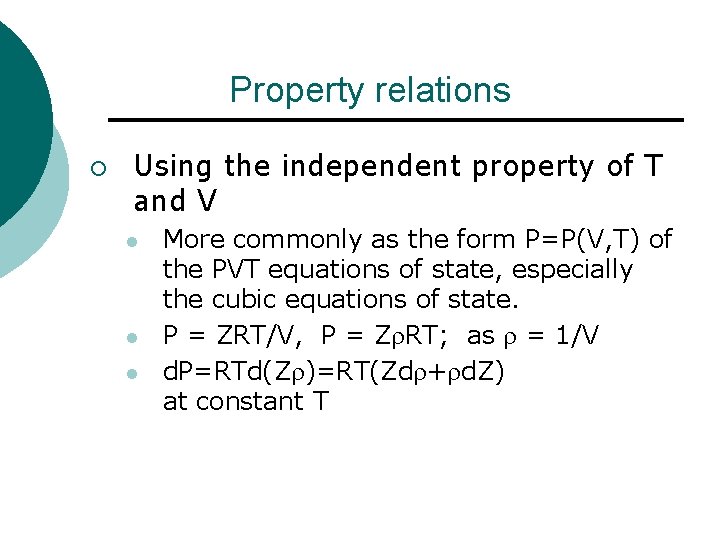
Property relations ¡ Using the independent property of T and V l l l More commonly as the form P=P(V, T) of the PVT equations of state, especially the cubic equations of state. P = ZRT/V, P = Z RT; as = 1/V d. P=RTd(Z )=RT(Zd + d. Z) at constant T

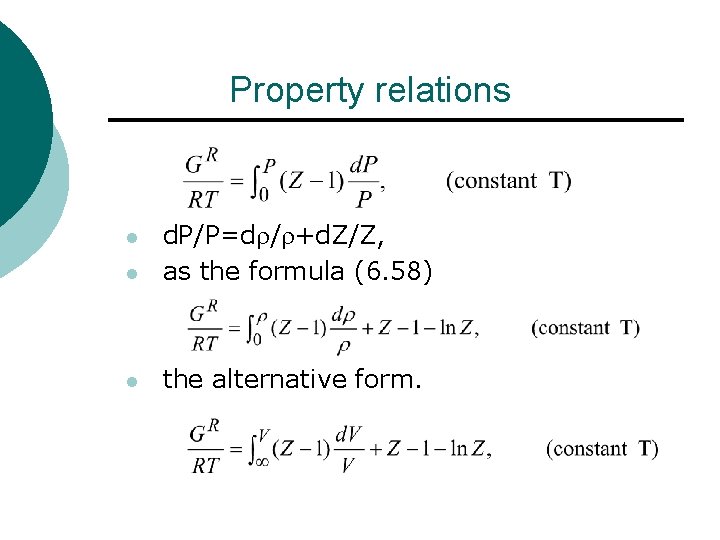
Property relations l d. P/P=d / +d. Z/Z, as the formula (6. 58) l the alternative form. l

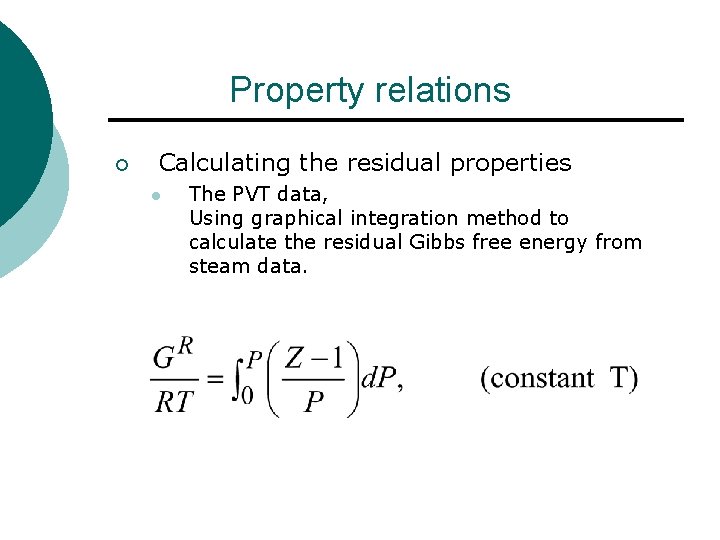
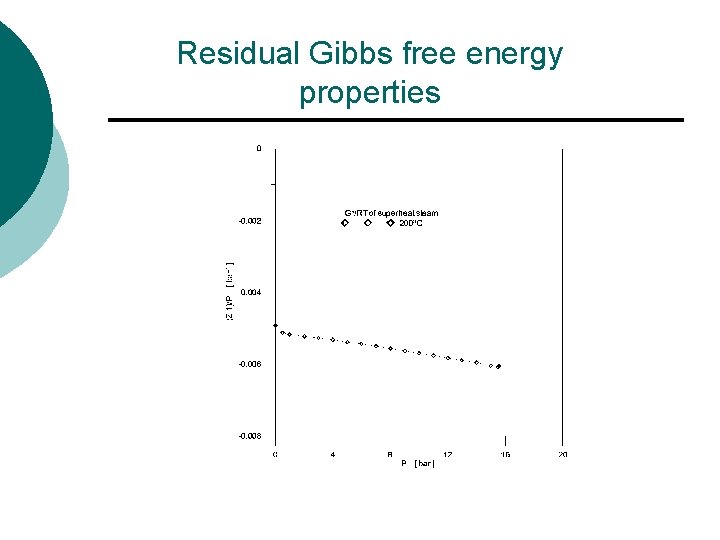
Property relations ¡ Calculating the residual properties l The PVT data, Using graphical integration method to calculate the residual Gibbs free energy from steam data.

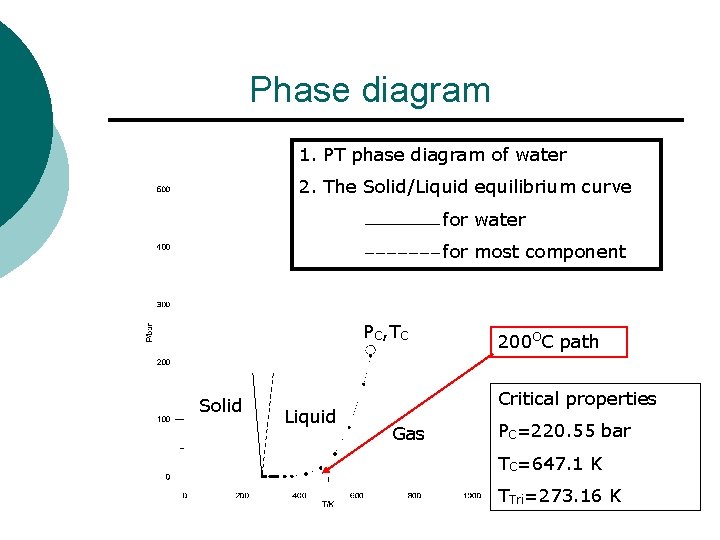
Phase diagram 1. PT phase diagram of water 2. The Solid/Liquid equilibrium curve for water for most component PC, TC Solid Liquid 200 OC path Critical properties Gas PC=220. 55 bar TC=647. 1 K TTri=273. 16 K

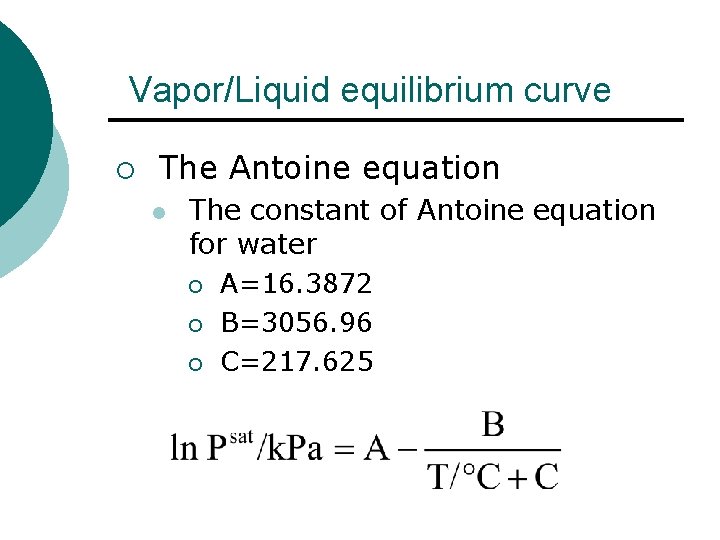
Vapor/Liquid equilibrium curve ¡ The Antoine equation l The constant of Antoine equation for water ¡ A=16. 3872 ¡ B=3056. 96 ¡ C=217. 625

Property ¡ Critical properties of water l PC=220. 55 bar l TC=647. 1 K l l l PTri=0. 611 k. Pa TTri=273. 16 K Tn=373. 15 K at P= 1. 01325 bar = 1 atm

Property relations ¡ Problems l Calculate the residual Gibbs energy of the 200 C superheat steam at 10 bar, using the PVT data from steam tables

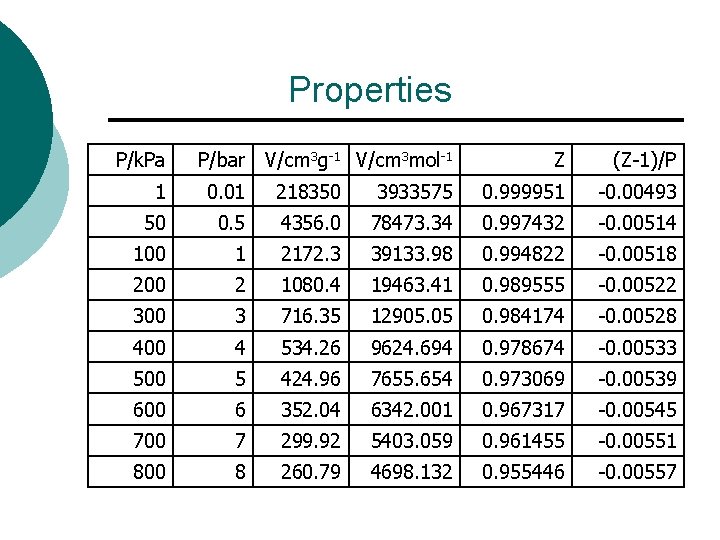
Properties P/k. Pa P/bar V/cm 3 g-1 V/cm 3 mol-1 Z (Z-1)/P 1 0. 01 218350 3933575 0. 999951 -0. 00493 50 0. 5 4356. 0 78473. 34 0. 997432 -0. 00514 100 1 2172. 3 39133. 98 0. 994822 -0. 00518 200 2 1080. 4 19463. 41 0. 989555 -0. 00522 300 3 716. 35 12905. 05 0. 984174 -0. 00528 400 4 534. 26 9624. 694 0. 978674 -0. 00533 500 5 424. 96 7655. 654 0. 973069 -0. 00539 600 6 352. 04 6342. 001 0. 967317 -0. 00545 700 7 299. 92 5403. 059 0. 961455 -0. 00551 800 8 260. 79 4698. 132 0. 955446 -0. 00557

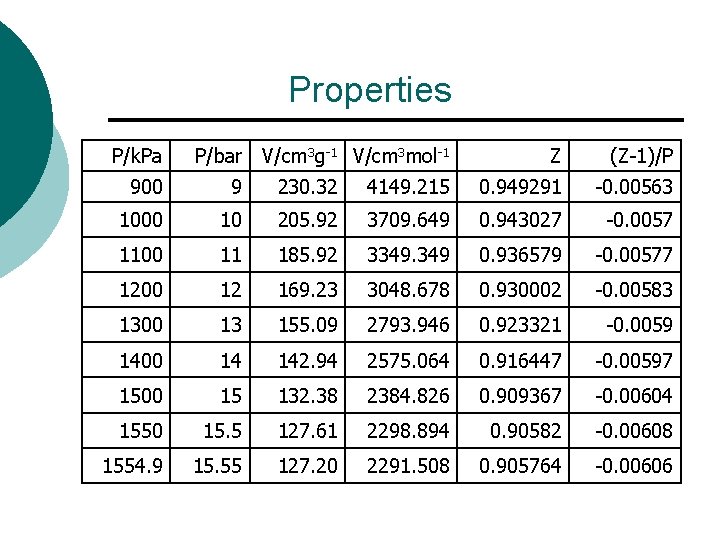
Properties P/k. Pa P/bar V/cm 3 g-1 V/cm 3 mol-1 Z (Z-1)/P 900 9 230. 32 4149. 215 0. 949291 -0. 00563 1000 10 205. 92 3709. 649 0. 943027 -0. 0057 1100 11 185. 92 3349. 349 0. 936579 -0. 00577 1200 12 169. 23 3048. 678 0. 930002 -0. 00583 1300 13 155. 09 2793. 946 0. 923321 -0. 0059 1400 14 142. 94 2575. 064 0. 916447 -0. 00597 1500 15 132. 38 2384. 826 0. 909367 -0. 00604 1550 15. 5 127. 61 2298. 894 0. 90582 -0. 00608 1554. 9 15. 55 127. 20 2291. 508 0. 905764 -0. 00606

Residual Gibbs free energy properties

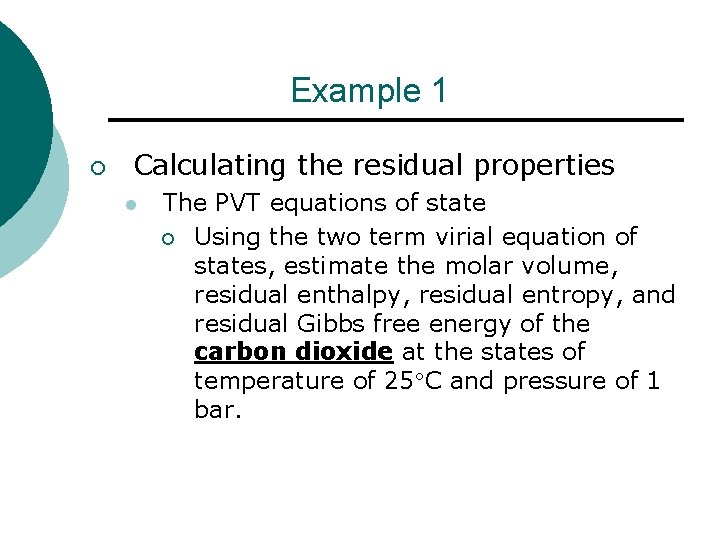
Example 1 ¡ Calculating the residual properties l The PVT equations of state ¡ Using the two term virial equation of states, estimate the molar volume, residual enthalpy, residual entropy, and residual Gibbs free energy of the carbon dioxide at the states of temperature of 25 C and pressure of 1 bar.

Deriving the formulas ¡ ¡ ¡ The equations of residual Gibbs free energy Three type of the two term virial equation The residual Gibbs free energy for a gas at each P and T

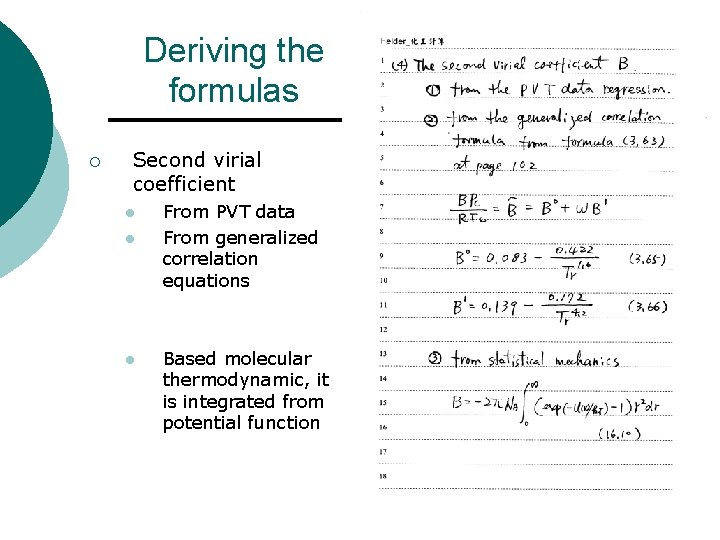
Deriving the formulas ¡ Second virial coefficient l l l From PVT data From generalized correlation equations Based molecular thermodynamic, it is integrated from potential function

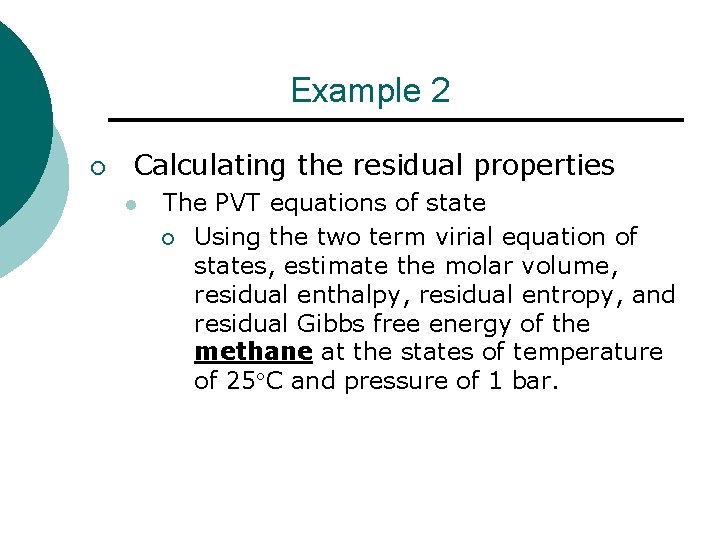
Example 2 ¡ Calculating the residual properties l The PVT equations of state ¡ Using the two term virial equation of states, estimate the molar volume, residual enthalpy, residual entropy, and residual Gibbs free energy of the methane at the states of temperature of 25 C and pressure of 1 bar.

Example 3 ¡ Calculating the residual properties l The PVT equations of state ¡ ( ?) Using the two term virial equation of states, estimate the molar volume, residual enthalpy, residual entropy, and residual Gibbs free energy of the benzene at the states of temperature of 25 C and pressure of 1 bar.

Theorem of corresponding states ¡ Two-parameter theorem of corresponding states l l Given T and P Finding Tc, Pc Calculate Tr, Pr Using Tr, Pr to finding the reduced properties from Table E. 1 to E. 16

Theorem of corresponding states ¡ Three-parameter theorem of corresponding states l l Given T and P Finding Tc, Pc, Calculate Tr, Pr Using Tr, Pr, to finding the reduced properties from Table E. 1 to E. 16

Theorem of corresponding states ¡ Based on the corresponding states principles l The Lee/Kesler generalized correlation tables, Appendix E at text books of pages from 695 to 711. l The properties of some pure species tables, Appendix B at text books of pages from 679 to 682.

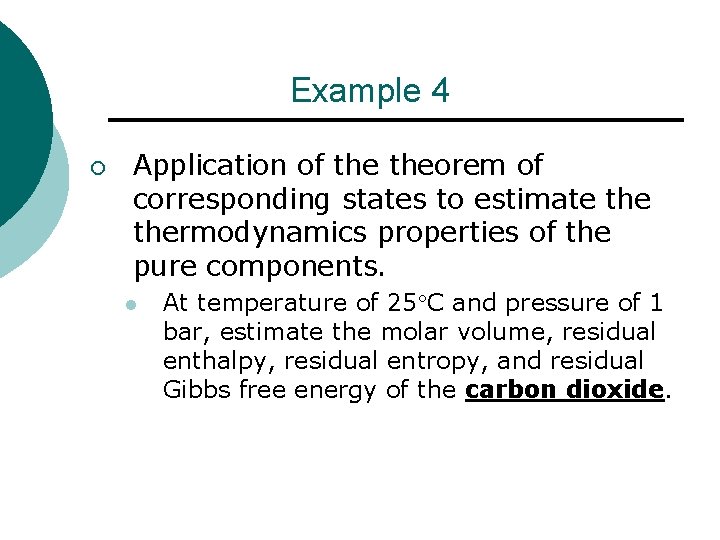
Example 4 ¡ Application of theorem of corresponding states to estimate thermodynamics properties of the pure components. l At temperature of 25 C and pressure of 1 bar, estimate the molar volume, residual enthalpy, residual entropy, and residual Gibbs free energy of the carbon dioxide.

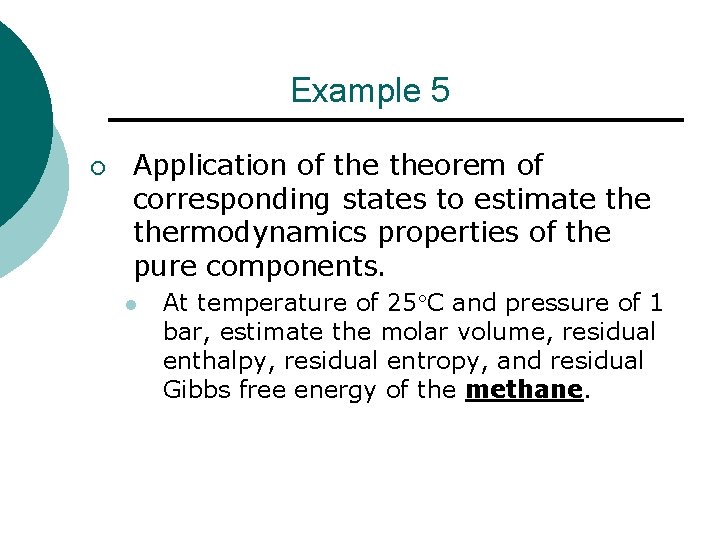
Example 5 ¡ Application of theorem of corresponding states to estimate thermodynamics properties of the pure components. l At temperature of 25 C and pressure of 1 bar, estimate the molar volume, residual enthalpy, residual entropy, and residual Gibbs free energy of the methane.

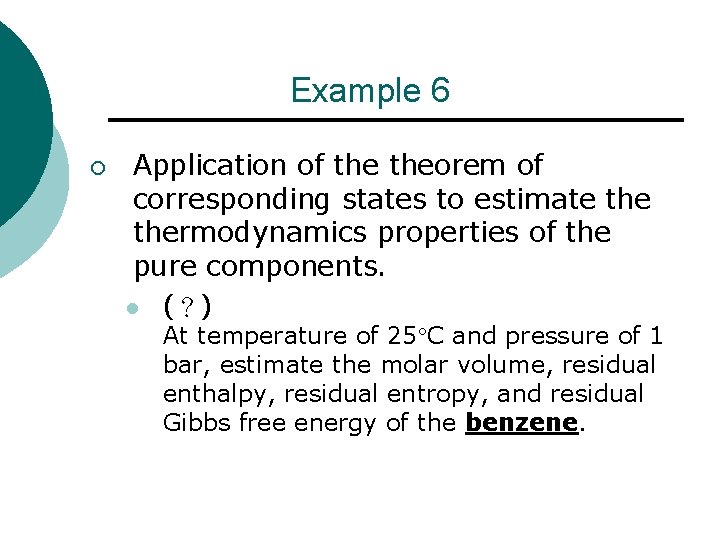
Example 6 ¡ Application of theorem of corresponding states to estimate thermodynamics properties of the pure components. l (?) At temperature of 25 C and pressure of 1 bar, estimate the molar volume, residual enthalpy, residual entropy, and residual Gibbs free energy of the benzene.