The Activity Series The Theory Behind the Activity

The Activity Series

The Theory Behind the Activity Series: Strong reducing agents require only weak oxidizing agents to react vigorously (eg. potassium reacts violently on contact with water. ) K (s) + H(OH) → H 2 (g) + KOH (aq) since K is higher than H on the activity series Potassium Video http: //www. instructables. com/id/Sodium-and-Potassium-In-Water/

Weak reducing agents will not react with even strong oxidizing agents (eg. part of the reason why gold is so precious is because it resists tarnishing. ) Au (s) + HCl (aq) –> no reaction This is because Au is NOT higher than H on the activity series Since it is NOT more reactive it can not displace the more reactive hydrogen from the compound

This is because metal atoms are by nature prone to losing electrons. However, some metals lose electrons more easily than others. Their readiness to be oxidized makes them powerful reducing agents. We can compare the power of reducing agents in the activity series for metals. Why do metals easily lose their electrons? ? Alkali metals have a very low ionization energy and thus their electrons are easily lost. Alkaline Earth metals also follow this trend and together they make up the top six metals on the activity series.
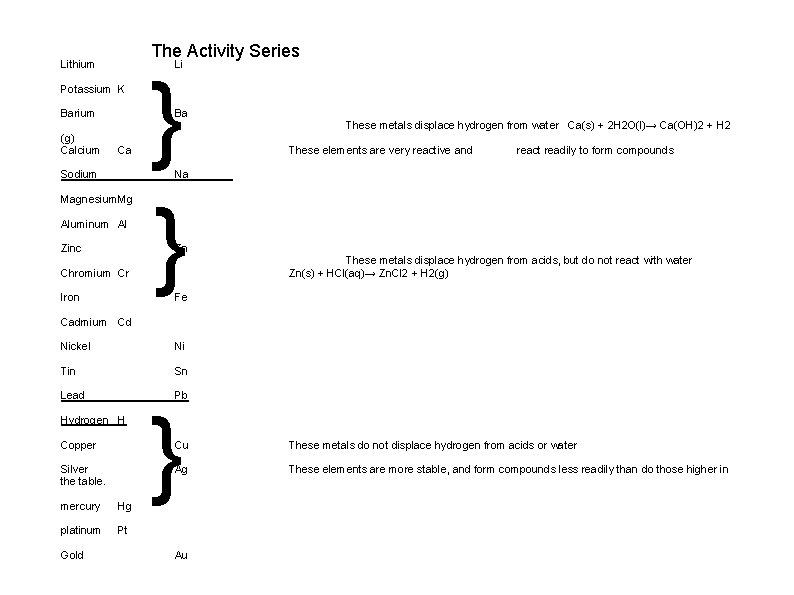
The Activity Series Lithium Li Potassium K Barium (g) Calcium } } Ba Ca Sodium These metals displace hydrogen from water Ca(s) + 2 H 2 O(l)→ Ca(OH)2 + H 2 These elements are very reactive and react readily to form compounds Na Magnesium. Mg Aluminum Al Zinc Zn Chromium Cr Iron These metals displace hydrogen from acids, but do not react with water Zn(s) + HCl(aq)→ Zn. Cl 2 + H 2(g) Fe Cadmium Cd Nickel Ni Tin Sn Lead Pb Hydrogen H Copper Silver the table. mercury Hg platinum Pt Gold } Cu These metals do not displace hydrogen from acids or water Ag These elements are more stable, and form compounds less readily than do those higher in Au
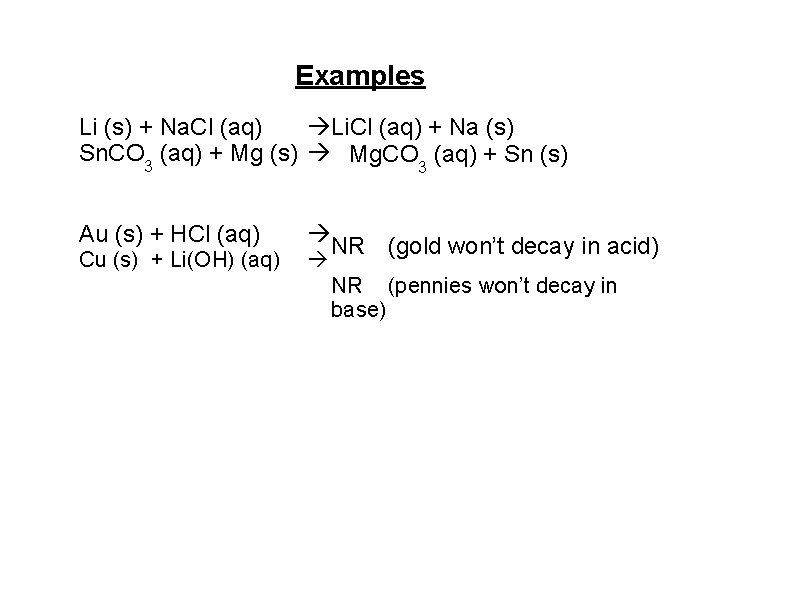
Examples Li (s) + Na. Cl (aq) Li. Cl (aq) + Na (s) Sn. CO 3 (aq) + Mg (s) Mg. CO 3 (aq) + Sn (s) Au (s) + HCl (aq) Cu (s) + Li(OH) (aq) NR (gold won’t decay in acid) NR (pennies won’t decay in base)
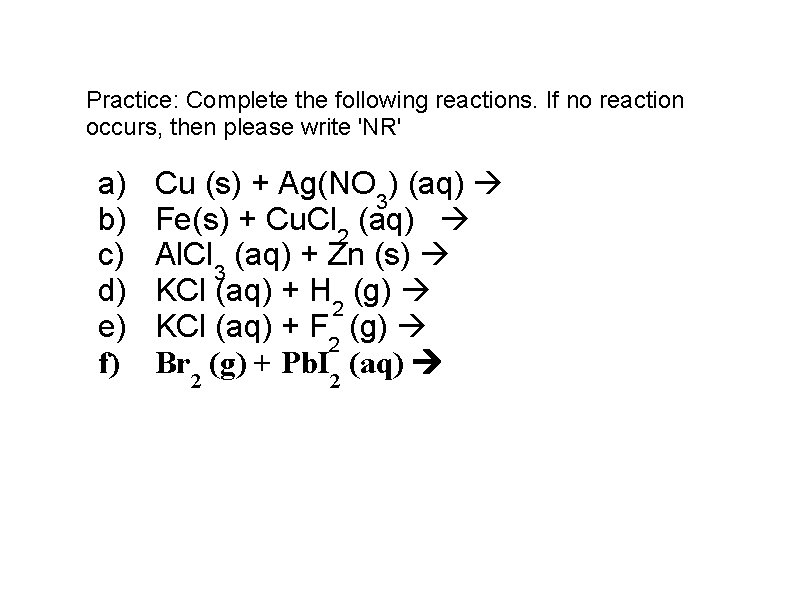
Practice: Complete the following reactions. If no reaction occurs, then please write 'NR' a) b) c) d) e) f) Cu (s) + Ag(NO 3) (aq) Fe(s) + Cu. Cl 2 (aq) Al. Cl 3 (aq) + Zn (s) KCl (aq) + H 2 (g) KCl (aq) + F 2 (g) Br 2 (g) + Pb. I 2 (aq)

g) h) I) j) k) l) m) n) o) p) q) lead + zinc acetate iron + aluminum oxide silver nitrate + nickel sodium bromide + iodine aluminum bromide + chlorine sodium iodide + bromine calcium + hydrochloric acid magnesium + nitric acid silver + sulfuric acid potassium + water sodium + water
- Slides: 8