The Activity Series Bromfield Honors Chemistry Objectives Introduce

The Activity Series Bromfield Honors Chemistry

Objectives Introduce the activity series for metals Introduce the activity series for halogens Use the activity series to predict if single replacement reactions occur

Reactivity Some elements are more reactive than others. Alkali water metals in

Comparing two sets of reactants Mg + Cu(NO 3)2 Cu + Mg(NO 3)2

Reactivity Some elements are more reactive than others. Some of this information has been listed in the activity series. Element Reactivity Li Rb K Ba Ca Na React with cold H 2 O and acids, replacing hydrogen Mg Al Mn Zn Cr Fe React with acids or steam, but usually not liquid water, to replace hydrogen Ni Sn Pb All react with acids, but not water, to replace hydrogen H 2 Cu Hg All react with oxygen to form oxides Ag Pt Au Mostly unreactive
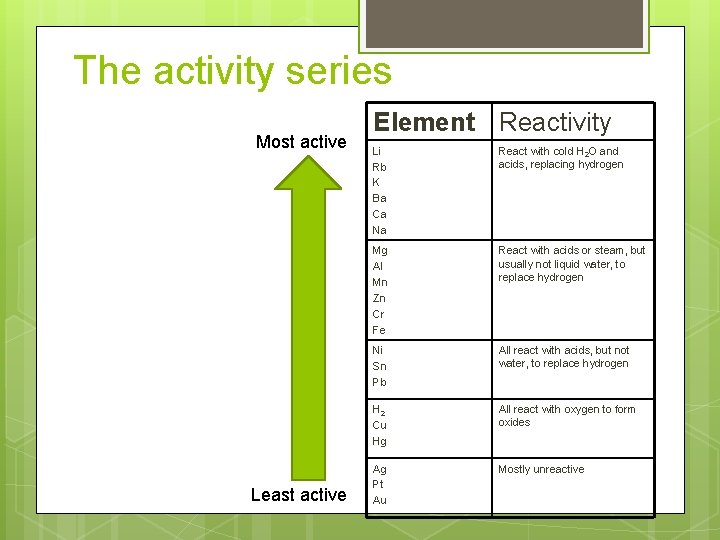
The activity series Most active Least active Element Reactivity Li Rb K Ba Ca Na React with cold H 2 O and acids, replacing hydrogen Mg Al Mn Zn Cr Fe React with acids or steam, but usually not liquid water, to replace hydrogen Ni Sn Pb All react with acids, but not water, to replace hydrogen H 2 Cu Hg All react with oxygen to form oxides Ag Pt Au Mostly unreactive

Using the activity series We can use the activity series to predict if a single replacement reaction will occur… Pattern:

Using the activity series We can use the activity series to predict if a single replacement reaction will occur… An element can replace an less active element in a compound

Using the activity series We can use the activity series to predict if a single replacement reaction will occur… An element can NOT replace a more active element in a compound
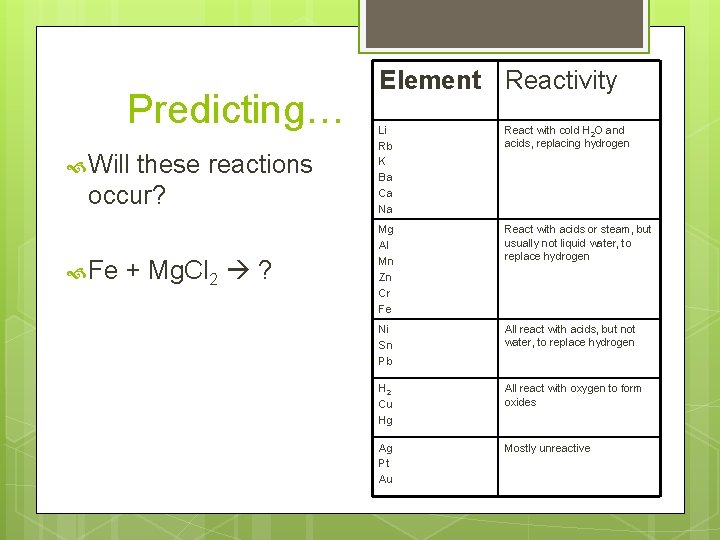
Predicting… Will these reactions occur? Fe + Mg. Cl 2 ? Element Reactivity Li Rb K Ba Ca Na React with cold H 2 O and acids, replacing hydrogen Mg Al Mn Zn Cr Fe React with acids or steam, but usually not liquid water, to replace hydrogen Ni Sn Pb All react with acids, but not water, to replace hydrogen H 2 Cu Hg All react with oxygen to form oxides Ag Pt Au Mostly unreactive

Predicting… Will these reactions occur? Li + Mg. Cl 2 ? Element Reactivity Li Rb K Ba Ca Na React with cold H 2 O and acids, replacing hydrogen Mg Al Mn Zn Cr Fe React with acids or steam, but usually not liquid water, to replace hydrogen Ni Sn Pb All react with acids, but not water, to replace hydrogen H 2 Cu Hg All react with oxygen to form oxides Ag Pt Au Mostly unreactive
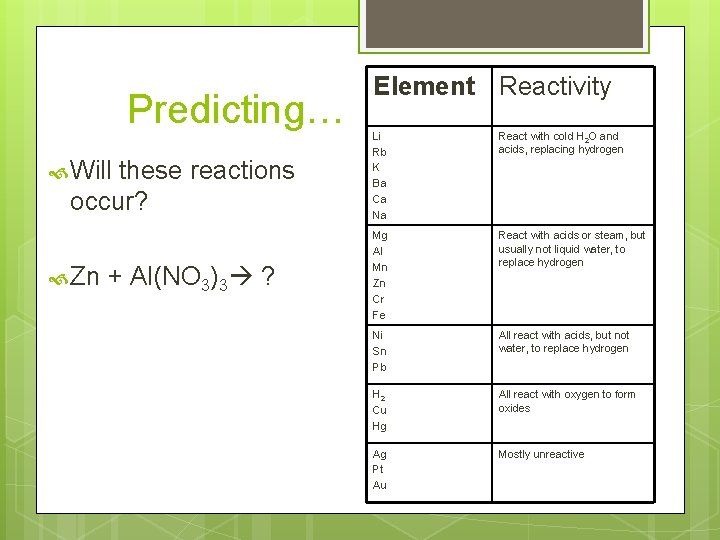
Predicting… Will these reactions occur? Zn + Al(NO 3)3 ? Element Reactivity Li Rb K Ba Ca Na React with cold H 2 O and acids, replacing hydrogen Mg Al Mn Zn Cr Fe React with acids or steam, but usually not liquid water, to replace hydrogen Ni Sn Pb All react with acids, but not water, to replace hydrogen H 2 Cu Hg All react with oxygen to form oxides Ag Pt Au Mostly unreactive
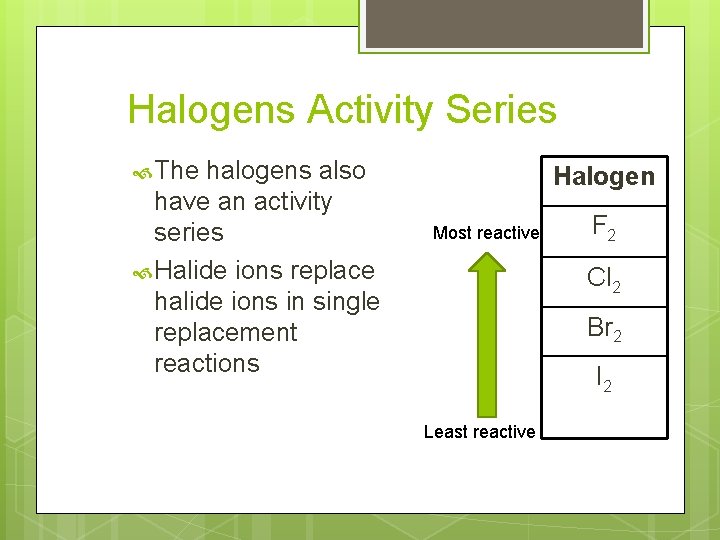
Halogens Activity Series The halogens also have an activity series Halide ions replace halide ions in single replacement reactions Halogen Most reactive F 2 Cl 2 Br 2 I 2 Least reactive

Halogens Activity Series Will the following reactions occur? Cl 2 + Na. F ? Halogen F 2 Cl 2 Br 2 I 2 most reactive least reactive

Halogens Activity Series Will the following reactions occur? Br 2 + AℓI 3 ? Halogen F 2 Cl 2 Br 2 I 2 most reactive least reactive
- Slides: 15