The 6 th School on Simulation and Modeling










- Slides: 30

The 6 th School on Simulation and Modeling Physics "Ab-initio methods and their applications" Hanoi, 27 - 29 November 2007 8 h 30 - 9 h 45 Tue. 27 Nov. Wed. 28 Nov. Thu. 29 Nov. Topical lecture Planets / High Pressure Topical lecture Atmospheric physics Methods: Ab-initio Molecular Dynamics 9 h 45 - 10 h 15 -11 h 30 Coffee break Methods Plane waves, cut-off, k-points, pseudopotentials, DFT, etc 11 h 30 – 13 h 30 – 16 h 00 Seminars Computer Lab 3 Lunch time Computer Lab 1 Computer Lab 2 Computer Lab 4

Ab-initio molecular dynamics in atmospheric science Sandro Scandolo The Abdus Salam International Center for Theoretical Physics Trieste, Italy www. ictp. it 6 th SMP, Hanoi, Nov 27 -29, 2007

Two case studies: Electron attachment at the surface of ice (the chemistry of the ozone hole) Infrared absorption by small water clusters (understanding the greenhouse effect)

Two case studies: Electron attachment at the surface of ice (the chemistry of the ozone hole) Infrared absorption by small water clusters (understanding the greenhouse effect)

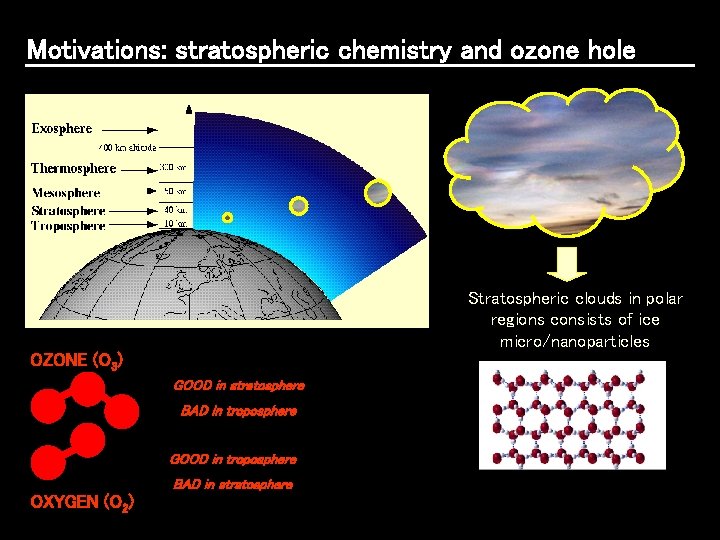
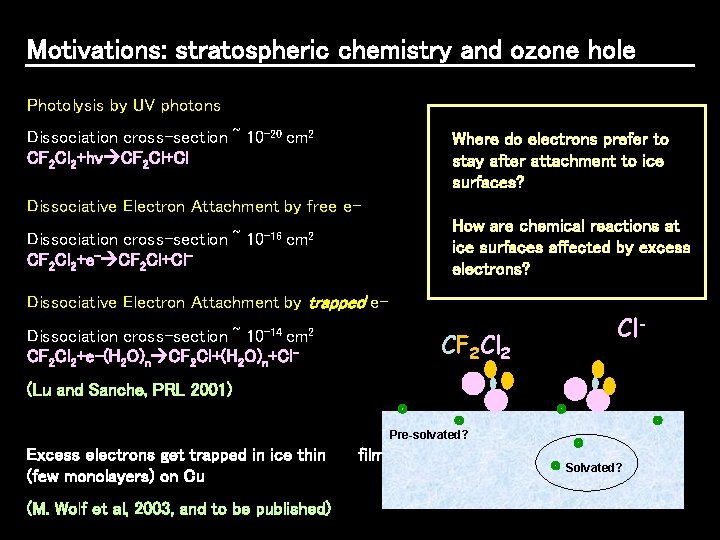
Motivations: stratospheric chemistry and ozone hole Stratospheric clouds in polar regions consists of ice micro/nanoparticles OZONE (O 3) GOOD in stratosphere BAD in troposphere GOOD in troposphere BAD in stratosphere OXYGEN (O 2)

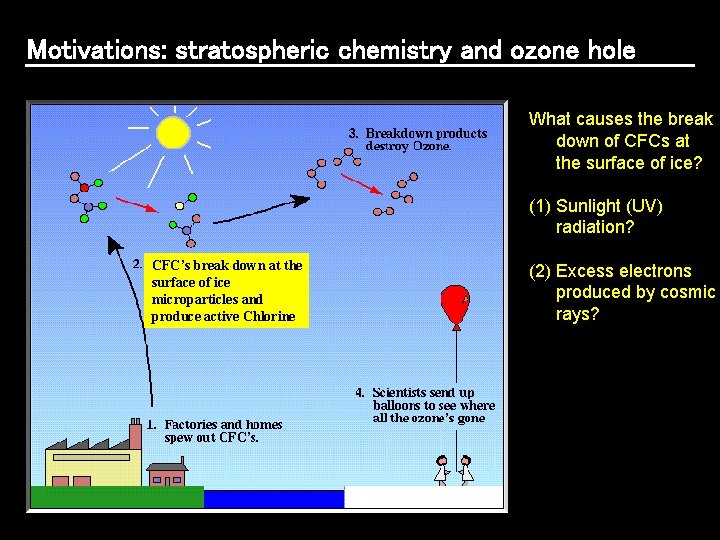
Motivations: stratospheric chemistry and ozone hole What causes the break down of CFCs at the surface of ice? (1) Sunlight (UV) radiation? CFC’s break down at the surface of ice microparticles and produce active Chlorine (2) Excess electrons produced by cosmic rays?

Motivations: stratospheric chemistry and ozone hole Photolysis by UV photons Dissociation cross-section ~ 10 -20 cm 2 CF 2 Cl 2+hv CF 2 Cl+Cl Where do electrons prefer to stay after attachment to ice surfaces? Dissociative Electron Attachment by free e. Dissociation cross-section ~ CF 2 Cl 2+e- CF 2 Cl+Cl- 10 -16 How are chemical reactions at ice surfaces affected by excess electrons? cm 2 Dissociative Electron Attachment by trapped e. Dissociation cross-section ~ 10 -14 cm 2 CF 2 Cl 2+e-(H 2 O)n CF 2 Cl+(H 2 O)n+Cl- CF 2 Cl 2 Cl- (Lu and Sanche, PRL 2001) Pre-solvated? Excess electrons get trapped in ice thin (few monolayers) on Cu (M. Wolf et al, 2003, and to be published) films Solvated?

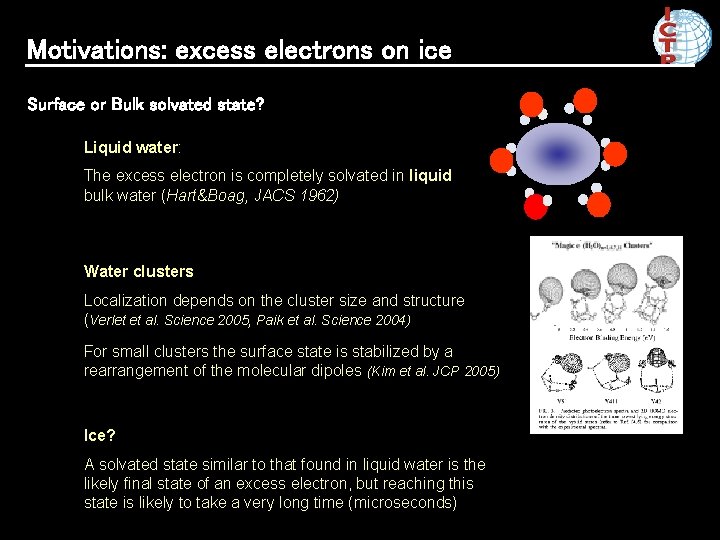
Motivations: excess electrons on ice Surface or Bulk solvated state? Liquid water: The excess electron is completely solvated in liquid bulk water (Hart&Boag, JACS 1962) Water clusters Localization depends on the cluster size and structure (Verlet et al. Science 2005, Paik et al. Science 2004) For small clusters the surface state is stabilized by a rearrangement of the molecular dipoles (Kim et al. JCP 2005) Ice? A solvated state similar to that found in liquid water is the likely final state of an excess electron, but reaching this state is likely to take a very long time (microseconds)

Level alignment at insulating surfaces Vacuum level energy Empty states Energy gap Filled states position

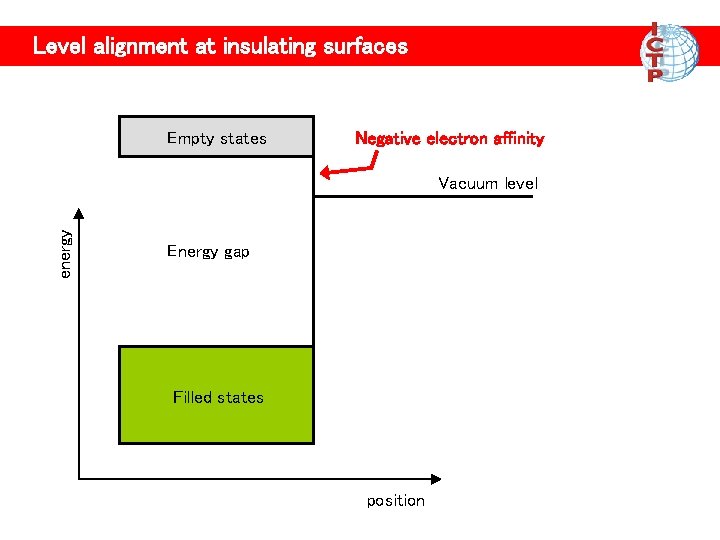
Level alignment at insulating surfaces Empty states Negative electron affinity energy Vacuum level Energy gap Filled states position


Convergence with vacuum thickness


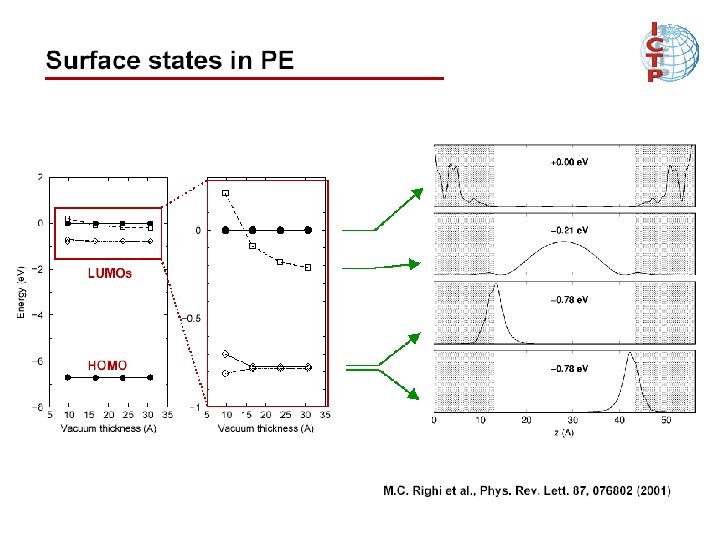
Surface states in polyethylene Surface in polyethylene M. C. Righi et al. , Phys. Rev. Lett. 87, 076802 (2001)

Ab-initio Molecular Dynamics 32 H 2 O molecules in Ih structure and complete proton disorder BLYP exchange-correlation functional and Martins-Troullier pseudopotentials Periodic boundary conditions Vacuum ~20 A Both neutral and charged (with excess electron) cases are considered Positive compensating background Self-interaction correction System evolved at T~150 K Where do excess e- prefer to stay? Do they self-trap as in liquid water?

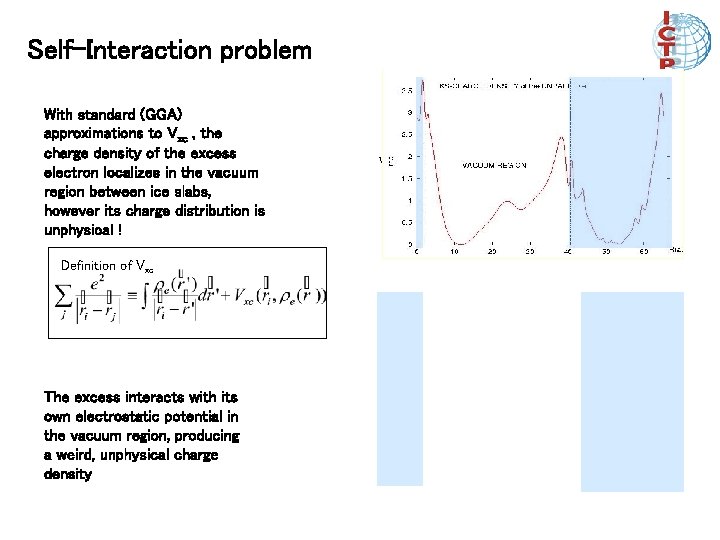
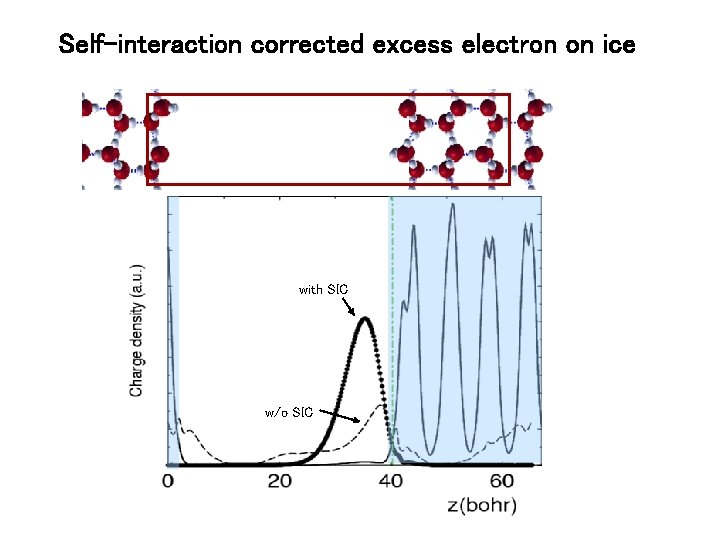
Self-Interaction problem With standard (GGA) approximations to Vxc , the charge density of the excess electron localizes in the vacuum region between ice slabs, however its charge distribution is unphysical ! Definition of Vxc The excess interacts with its own electrostatic potential in the vacuum region, producing a weird, unphysical charge density with SIC w/o SIC

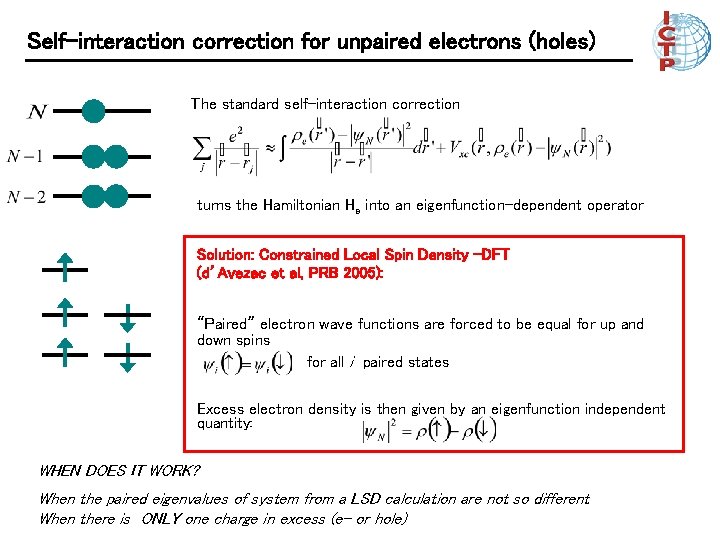
Self-interaction correction for unpaired electrons (holes) The standard self-interaction correction turns the Hamiltonian He into an eigenfunction-dependent operator Solution: Constrained Local Spin Density –DFT (d’Avezac et al, PRB 2005): “Paired” electron wave functions are forced to be equal for up and down spins for all i paired states Excess electron density is then given by an eigenfunction independent quantity: WHEN DOES IT WORK? When the paired eigenvalues of system from a LSD calculation are not so different When there is ONLY one charge in excess (e- or hole)

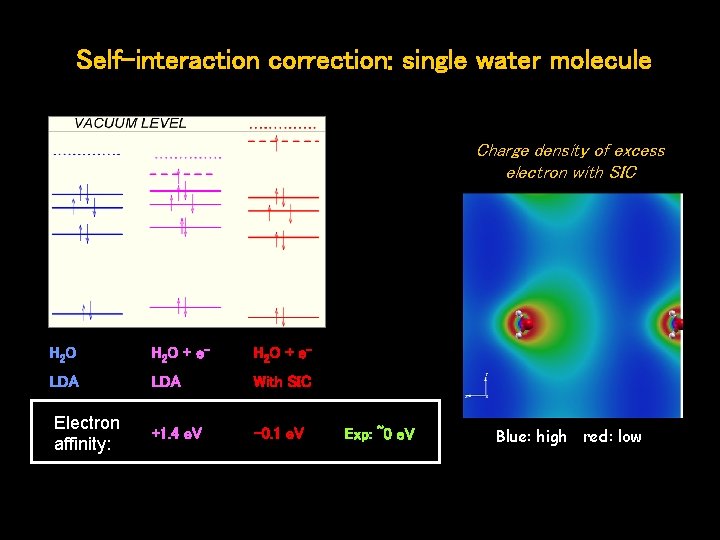
Self-interaction correction: single water molecule Charge density of excess electron with SIC H 2 O + e- LDA With SIC Electron affinity: +1. 4 e. V -0. 1 e. V Exp: ~0 e. V Blue: high red: low

Self-interaction corrected excess electron on ice with SIC w/o SIC

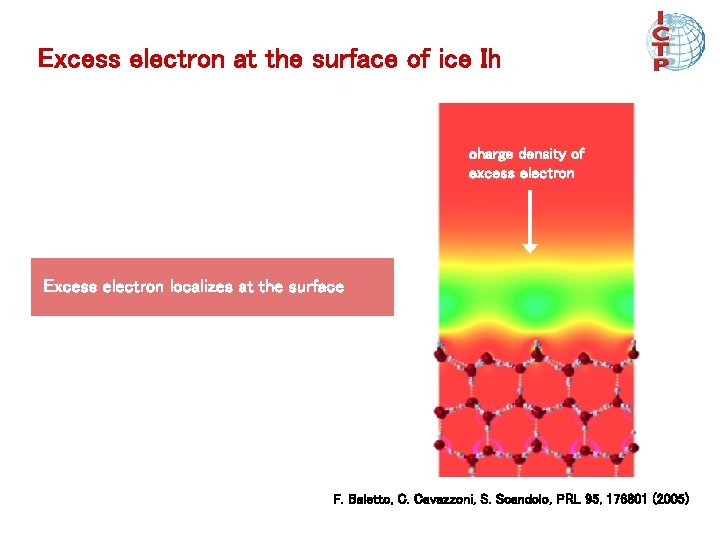
Excess electron at the surface of ice Ih charge density of excess electron Excess electron localizes at the surface F. Baletto, C. Cavazzoni, S. Scandolo, PRL 95, 176801 (2005)

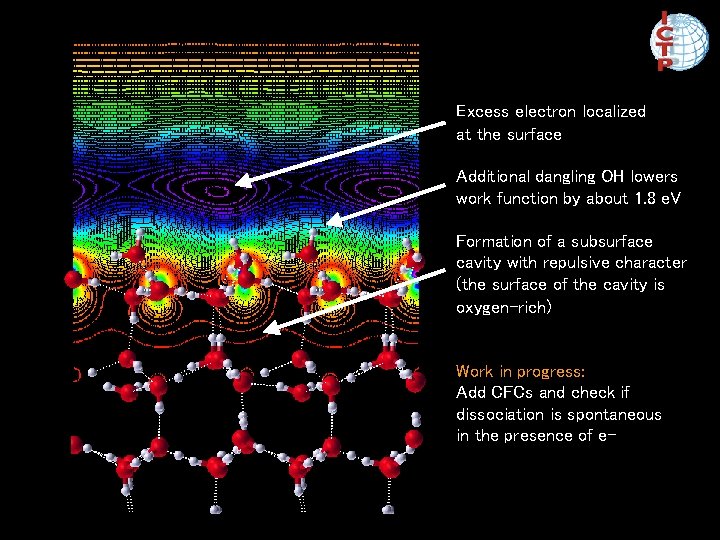
Neutral surface evolved for 1. 6 ps Surface with excess electron evolved for 2. 4 ps Additional dangling OH

Excess electron localized at the surface Additional dangling OH lowers work function by about 1. 8 e. V Formation of a subsurface cavity with repulsive character (the surface of the cavity is oxygen-rich) Work in progress: Add CFCs and check if dissociation is spontaneous in the presence of e-

Two case studies: Electron attachment at the surface of ice (the chemistry of the ozone hole) Infrared absorption by small water clusters (understanding the greenhouse effect)


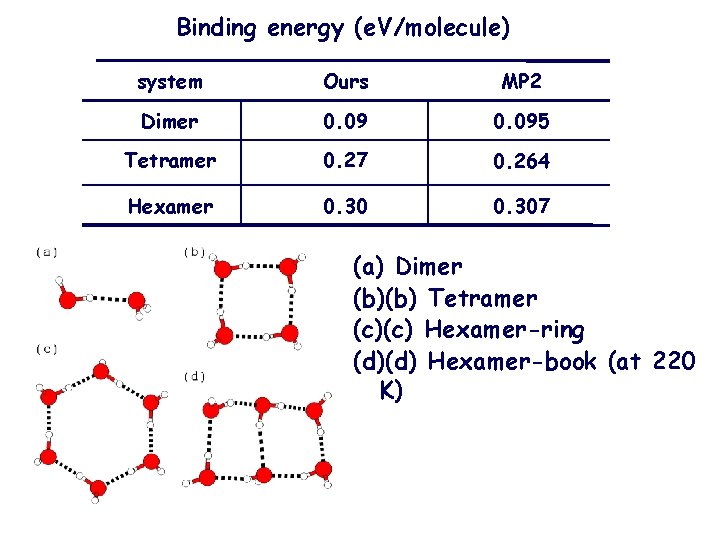
Binding energy (e. V/molecule) system Ours MP 2 Dimer 0. 095 Tetramer 0. 27 0. 264 Hexamer 0. 307 (a) Dimer (b)(b) Tetramer (c)(c) Hexamer-ring (d)(d) Hexamer-book (at 220 K)

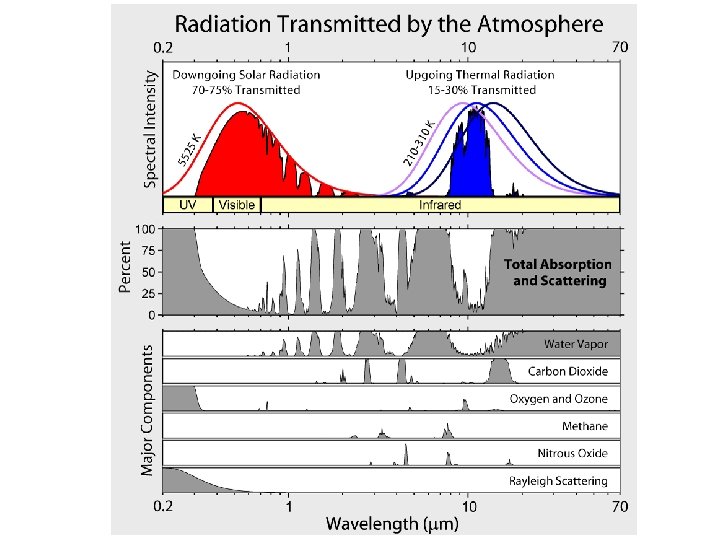
Water vapor absorption is completely different from bulk ice/water Water vapor 400 800 1200

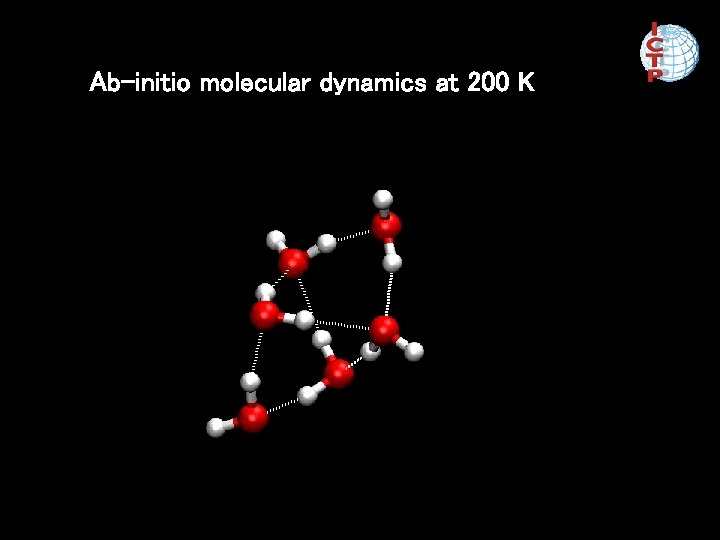
Ab-initio molecular dynamics at 200 K

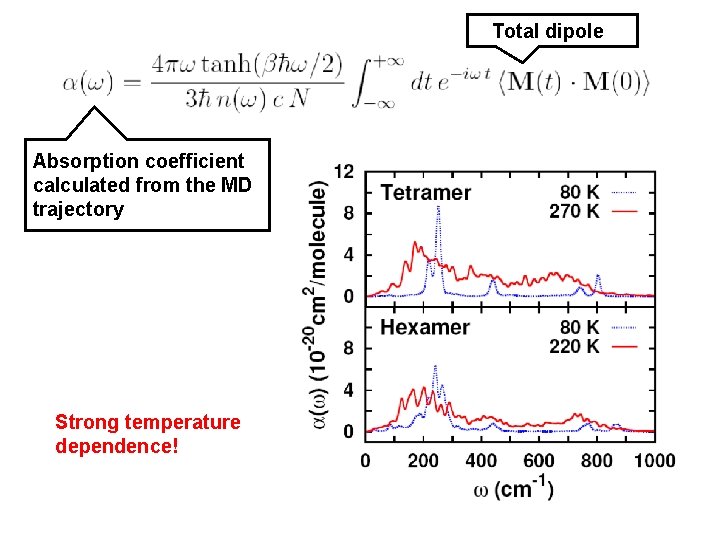
Total dipole Absorption coefficient calculated from the MD trajectory Strong temperature dependence!

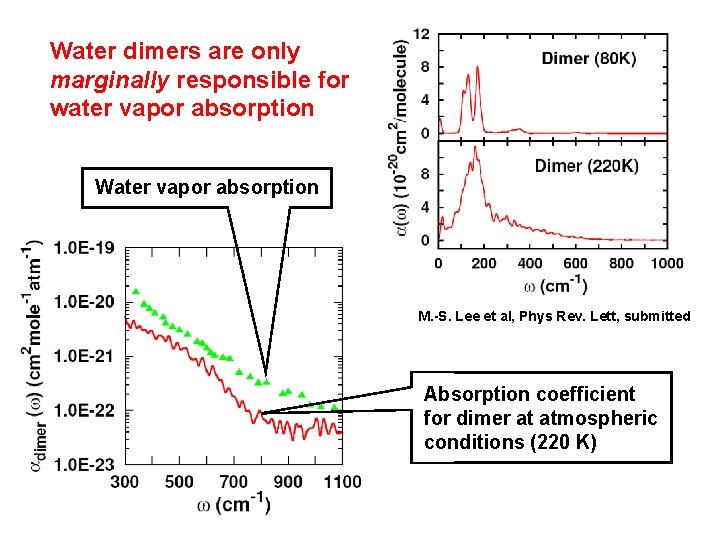
Water dimers are only marginally responsible for water vapor absorption Water vapor absorption M. -S. Lee et al, Phys Rev. Lett, submitted Absorption coefficient for dimer at atmospheric conditions (220 K)

Thanks to Francesca Baletto (now at King’s College London) Mal-Soon Lee (ICTP) Carlo Cavazzoni (CINECA, Bologna) Diep Quang Vinh (now at Purdue, USA)