The 5 Best Trials From 2015 That May

The 5 Best Trials From 2015 That May Impact My Practice Keynote Lecture David R. Holmes, Jr. , M. D. Mayo Clinic, Rochester CRT 2016 Washington, D. C. February 2015 © 2012 MFMER | slide-1

Presenter Disclosure Information David R. Holmes, Jr. , M. D. “The 5 Best Trials From 2015 That May Impact my Practice – Keynote Lecture” The following relationships exist related to this presentation: Both Mayo and I have technology that has been licensed to Boston Scientific concerning LAA occlusion © 2012 MFMER | slide-4

Change how we think Expand our frontiers Change what we do and how we do it Change how we respond to the environment Change how we design subsequent trials © 2012 MFMER | slide-6
“Well, I’ve got your final grades ready, although I’m afraid not everyone here will be moving up. ” © 2012 MFMER | slide-8

© 2012 MFMER | slide-12
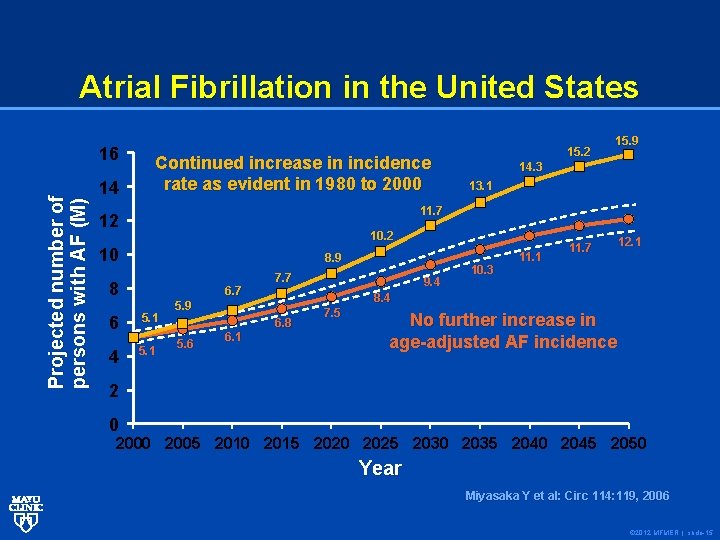
Atrial Fibrillation in the United States Projected number of persons with AF (M) 16 14 Continued increase in incidence rate as evident in 1980 to 2000 15. 2 15. 9 14. 3 13. 1 11. 7 12 10 8. 9 8 6. 7 6 5. 1 4 5. 1 7. 7 6. 1 11. 1 12. 1 8. 4 5. 9 5. 6 9. 4 10. 3 11. 7 6. 8 7. 5 No further increase in age-adjusted AF incidence 2 0 2005 2010 2015 2020 2025 2030 2035 2040 2045 2050 Year Miyasaka Y et al: Circ 114: 119, 2006 © 2012 MFMER | slide-15
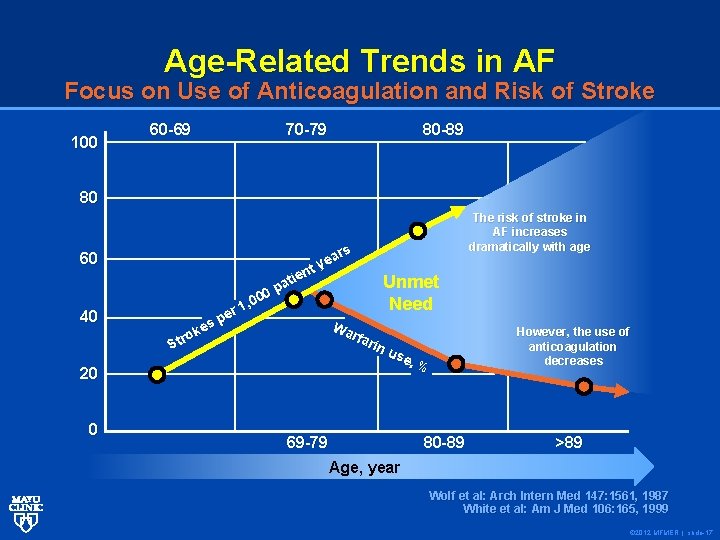
Age-Related Trends in AF Focus on Use of Anticoagulation and Risk of Stroke 100 60 -69 70 -79 80 -89 80 60 40 20 0 ars e y nt e i t Unmet pa 0 0 , 0 Need r 1 e p Wa es k rfa o r rin St us e, % 69 -79 80 -89 The risk of stroke in AF increases dramatically with age However, the use of anticoagulation decreases >89 Age, year Wolf et al: Arch Intern Med 147: 1561, 1987 White et al: Am J Med 106: 165, 1999 © 2012 MFMER | slide-17
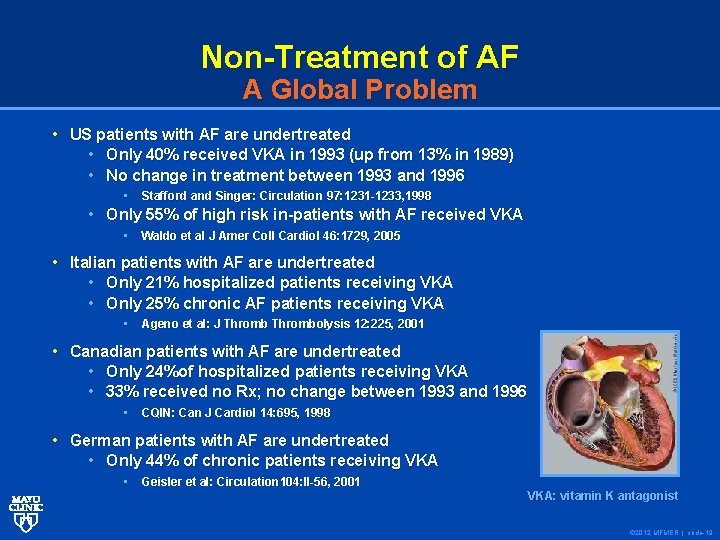
Non-Treatment of AF A Global Problem • US patients with AF are undertreated • Only 40% received VKA in 1993 (up from 13% in 1989) • No change in treatment between 1993 and 1996 • Stafford and Singer: Circulation 97: 1231 -1233, 1998 • Only 55% of high risk in-patients with AF received VKA • Waldo et al J Amer Coll Cardiol 46: 1729, 2005 Waldo et al • Italian patients with AF are undertreated • Only 21% hospitalized patients receiving VKA • Only 25% chronic AF patients receiving VKA • Ageno et al: J Thrombolysis 12: 225, 2001 Ageno et al: • Canadian patients with AF are undertreated • Only 24%of hospitalized patients receiving VKA • 33% received no Rx; no change between 1993 and 1996 • CQIN: Can J Cardiol 14: 695, 1998 CQIN: Can J Cardiol • German patients with AF are undertreated • Only 44% of chronic patients receiving VKA • Geisler et al: Circulation 104: II-56, 2001 VKA: vitamin K antagonist © 2012 MFMER | slide-19
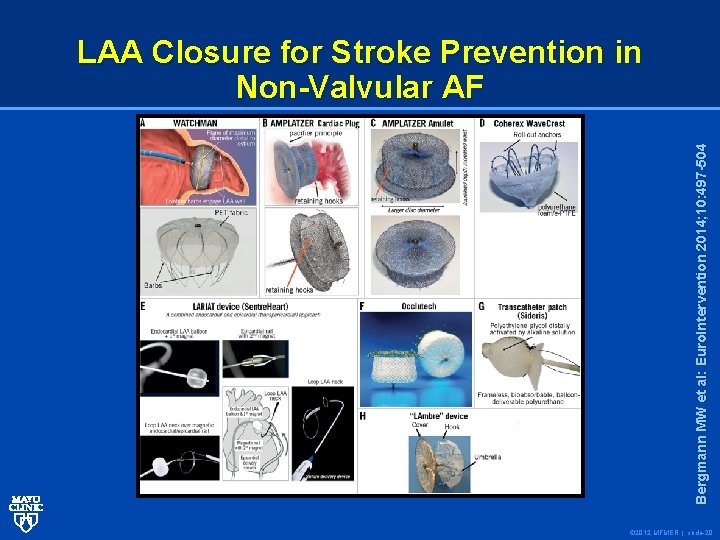
Bergmann MW et al: Euro. Intervention 2014; 10: 497 -504 LAA Closure for Stroke Prevention in Non-Valvular AF © 2012 MFMER | slide-20
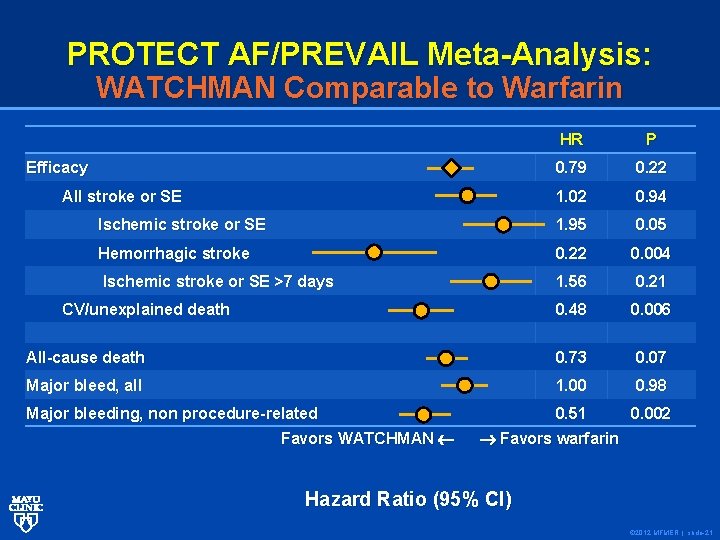
PROTECT AF/PREVAIL Meta-Analysis: WATCHMAN Comparable to Warfarin HR P 0. 79 0. 22 1. 02 0. 94 Ischemic stroke or SE 1. 95 0. 05 Hemorrhagic stroke 0. 22 0. 004 Ischemic stroke or SE >7 days 1. 56 0. 21 0. 48 0. 006 All-cause death 0. 73 0. 07 Major bleed, all 1. 00 0. 98 Major bleeding, non procedure-related 0. 51 0. 002 Efficacy All stroke or SE CV/unexplained death Favors WATCHMAN Favors warfarin Hazard Ratio (95% CI) © 2012 MFMER | slide-21
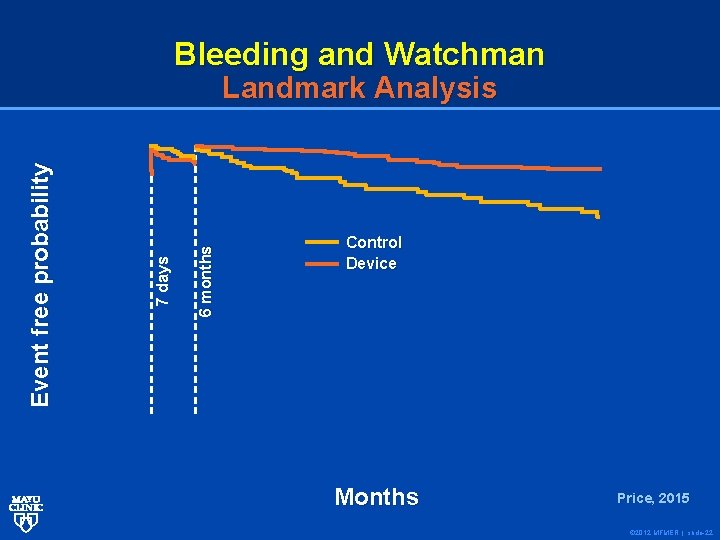
Bleeding and Watchman 6 months 7 days Event free probability Landmark Analysis Control Device Months Price, 2015 © 2012 MFMER | slide-22

Conclusions: In patients with NVAF at increased risk for stroke or bleeding who are candidates for chronic anticoagulation, LAAC resulted in improved rates of hemorrhagic stroke, cardiovascular/unexplained death, and nonprocedural bleeding compared to warfarin. © 2012 MFMER | slide-23

• The Centers for Medicare & Medicaid Services (CMS) covers percutaneous left atrial appendage closure (LAAC) for non-valvular atrial fibrillation (NVAF) through Coverage with Evidence Development (CED) under 1862(a)(1)(E) of the Social Security Act with the following conditions: • LAA closure devices are covered when the device has received Food & Drug Administration (FDA) Premarket Approval (PMA) for that device’s FDA-approved indication and meet all of the conditions specified below: • CHADS 2 – ≥ 2 • CHADS 2 -VASc – ≥ 3 • Shared decision making with independent noninterventional MD • Suitable for short-term AC but deemed unable or unsuitable for long-term • Trained physicians • Enrolled in a registry © 2012 MFMER | slide-25

Hair Today, Gone Tomorrow © 2012 MFMER | slide-26

Here Today, Gone Tomorrow 1) Provide mechanical support early • Prevent recoil • Rx dissections 2) Drug delivery • Prevent tissue regrowth 3) Disappearance of entire structure • Facilitate return to normal physiology 4) Allow access to distal coronary beds • Enable potential for surgical revascularization © 2012 MFMER | slide-27
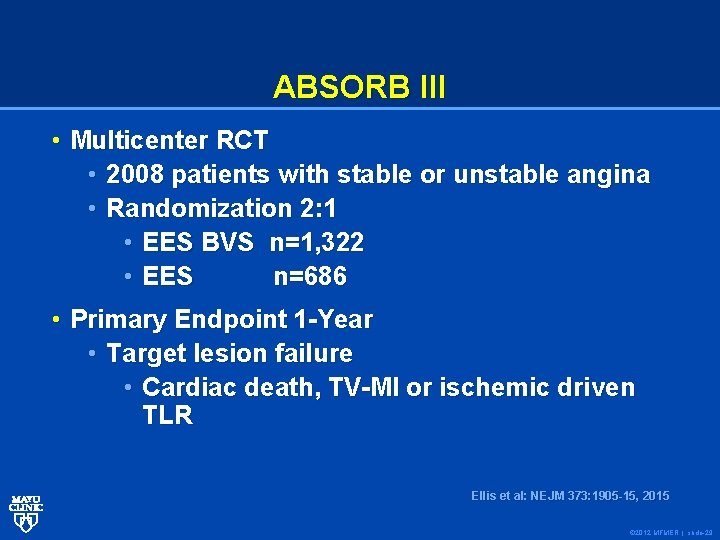
ABSORB III • Multicenter RCT • 2008 patients with stable or unstable angina • Randomization 2: 1 • EES BVS n=1, 322 • EES n=686 • Primary Endpoint 1 -Year • Target lesion failure • Cardiac death, TV-MI or ischemic driven TLR Ellis et al: NEJM 373: 1905 -15, 2015 © 2012 MFMER | slide-29
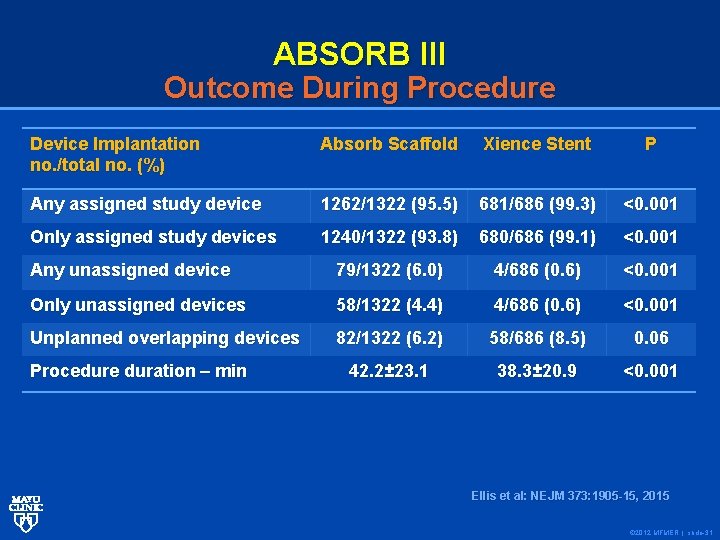
ABSORB III Outcome During Procedure Device Implantation no. /total no. (%) Absorb Scaffold Xience Stent P Any assigned study device 1262/1322 (95. 5) 681/686 (99. 3) <0. 001 Only assigned study devices 1240/1322 (93. 8) 680/686 (99. 1) <0. 001 Any unassigned device 79/1322 (6. 0) 4/686 (0. 6) <0. 001 Only unassigned devices 58/1322 (4. 4) 4/686 (0. 6) <0. 001 Unplanned overlapping devices 82/1322 (6. 2) 58/686 (8. 5) 0. 06 42. 2± 23. 1 38. 3± 20. 9 <0. 001 Procedure duration – min Ellis et al: NEJM 373: 1905 -15, 2015 © 2012 MFMER | slide-31
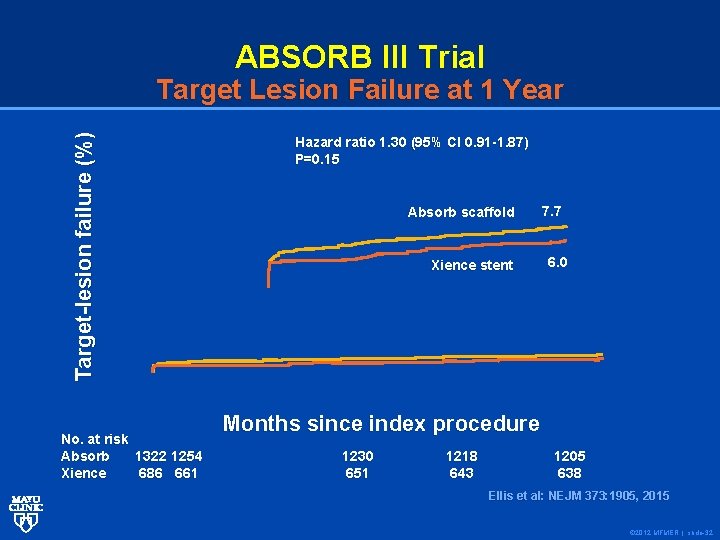
ABSORB III Trial Target-lesion failure (%) Target Lesion Failure at 1 Year No. at risk Absorb 1322 1254 Xience 686 661 Hazard ratio 1. 30 (95% CI 0. 91 -1. 87) P=0. 15 Absorb scaffold Xience stent 7. 7 6. 0 Months since index procedure 1230 651 1218 643 1205 638 Ellis et al: : NEJM 373: 1905, 2015 Ellis et al © 2012 MFMER | slide-32
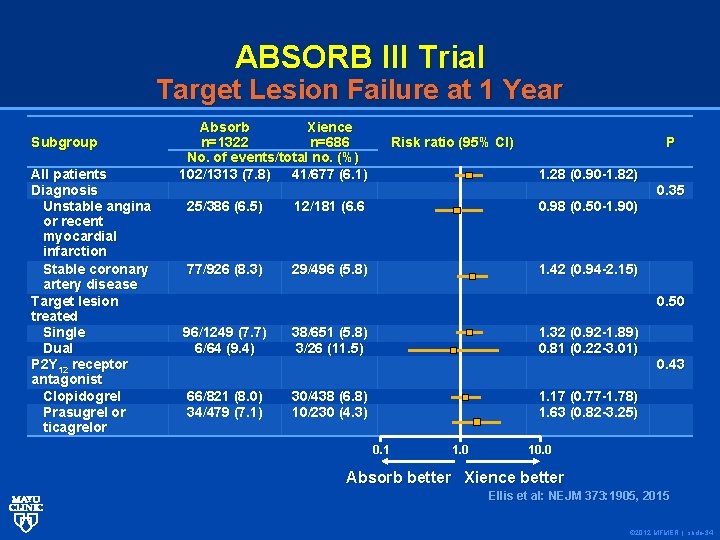
ABSORB III Trial Target Lesion Failure at 1 Year Subgroup All patients Diagnosis Unstable angina or recent myocardial infarction Stable coronary artery disease Target lesion treated Single Dual P 2 Y 12 receptor antagonist Clopidogrel Prasugrel or ticagrelor Absorb Xience n=1322 n=686 No. of events/total no. (%) 102/1313 (7. 8) 41/677 (6. 1) Risk ratio (95% CI) P 1. 28 (0. 90 -1. 82) 25/386 (6. 5) 12/181 (6. 6 0. 98 (0. 50 -1. 90) 77/926 (8. 3) 29/496 (5. 8) 1. 42 (0. 94 -2. 15) 0. 35 0. 50 96/1249 (7. 7) 6/64 (9. 4) 38/651 (5. 8) 3/26 (11. 5) 1. 32 (0. 92 -1. 89) 0. 81 (0. 22 -3. 01) 66/821 (8. 0) 34/479 (7. 1) 30/438 (6. 8) 10/230 (4. 3) 1. 17 (0. 77 -1. 78) 1. 63 (0. 82 -3. 25) 0. 1 1. 0 0. 43 10. 0 Absorb better Xience better Ellis et al: NEJM 373: 1905, 2015 Ellis et al: NEJM 373: 1905, 2015 © 2012 MFMER | slide-34
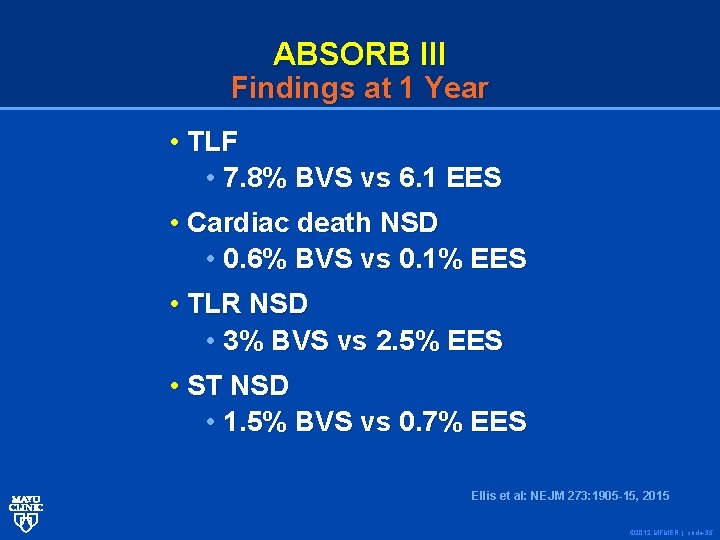
ABSORB III Findings at 1 Year • TLF • 7. 8% BVS vs 6. 1 EES • Cardiac death NSD • 0. 6% BVS vs 0. 1% EES • TLR NSD • 3% BVS vs 2. 5% EES • ST NSD • 1. 5% BVS vs 0. 7% EES Ellis et al: NEJM 2 73: 1905 -15, 2015 Ellis et al: NEJM 273: 1905 -15, 2015 © 2012 MFMER | slide-35

CONCLUSIONS In this large-scale, randomized trial, treatment of noncomplex obstructive coronary artery disease with an everolimuseluting bioresorbable vascular scaffold, as compared with an everolimus eluting cobalt–chromium stent, was within the prespecified margin for noninferiority with respect to targetlesion failure at 1 year. © 2012 MFMER | slide-36

Biovascular Scaffolds When You Should Consider • Never • Rarely • Sometimes • Often © 2012 MFMER | slide-37

© 2012 MFMER | slide-49

© 2012 MFMER | slide-50

Definition of “Firestorm” A firestorm is a usually stationary fire which attains such intensity that it creates and sustains its own wind system. It is most commonly a natural phenomenon, created during some of the largest bushfires, forest fires, and wildfires. The Black Saturday bushfires, the Great Peshtigo Fire and the Ash Wednesday fires are examples of firestorms. Firestorms can also be deliberate effects of targeted explosives such as occurred as a result of the aerial bombings of Dresden, Hamburg, Stalingrad, Tokyo, the atomic bombing of Hiroshima and Nagasaki and The Blitz during World War II. © 2012 MFMER | slide-51
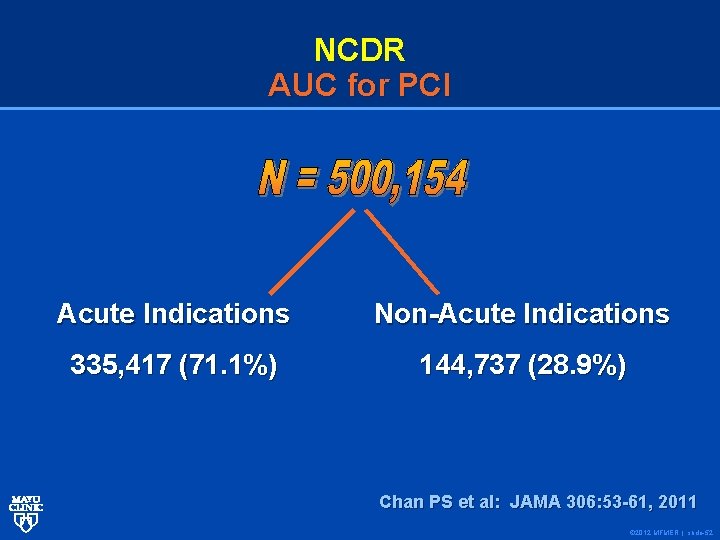
NCDR AUC for PCI Acute Indications Non-Acute Indications 335, 417 (71. 1%) 144, 737 (28. 9%) Chan PS et al: JAMA 306: 53 -61, 2011 © 2012 MFMER | slide-52
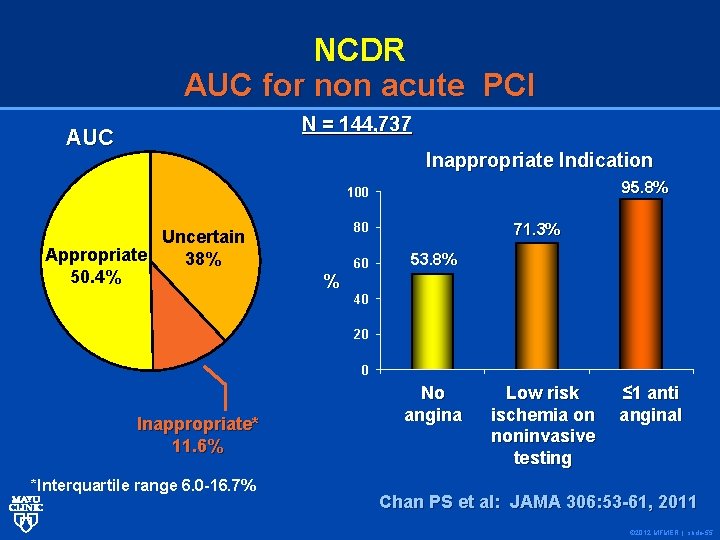
NCDR AUC for non acute PCI N = 144, 737 AUC Inappropriate Indication 95. 8% 100 Uncertain Appropriate 38% 50. 4% 80 % 60 71. 3% 53. 8% 40 20 0 Inappropriate* 11. 6% *Interquartile range 6. 0 -16. 7% No angina Low risk ischemia on noninvasive testing ≤ 1 anti anginal Chan PS et al: JAMA 306: 53 -61, 2011 © 2012 MFMER | slide-55
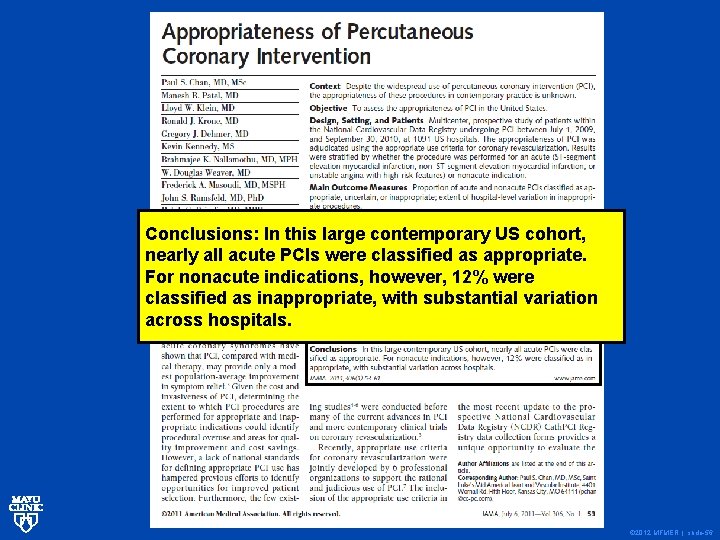
Conclusions: In this large contemporary US cohort, nearly all acute PCIs were classified as appropriate. For nonacute indications, however, 12% were classified as inappropriate, with substantial variation across hospitals. © 2012 MFMER | slide-56
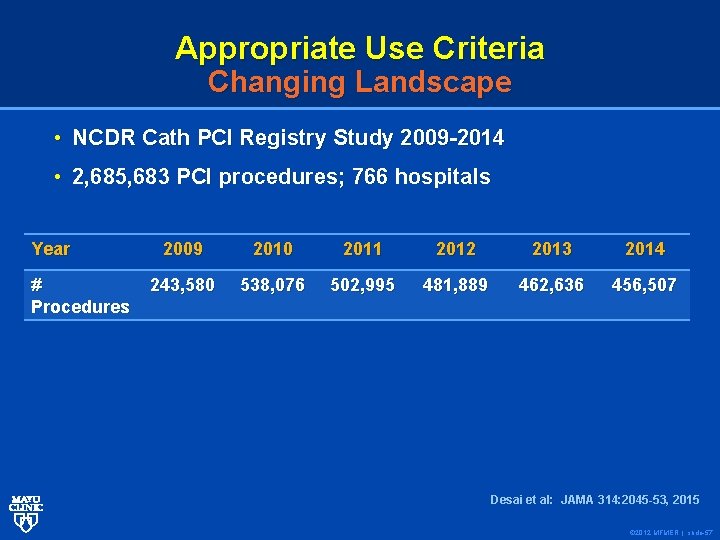
Appropriate Use Criteria Changing Landscape • NCDR Cath PCI Registry Study 2009 -2014 • 2, 685, 683 PCI procedures; 766 hospitals Year # Procedures 2009 2010 2011 2012 2013 2014 243, 580 538, 076 502, 995 481, 889 462, 636 456, 507 Desai et al: JAMA 314: 2045 -53, 2015 © 2012 MFMER | slide-57
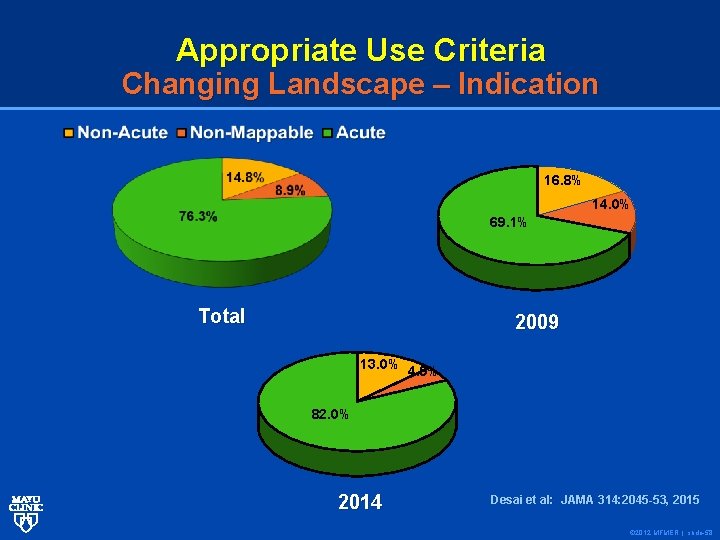
Appropriate Use Criteria Changing Landscape – Indication 16. 8% 14. 0% 69. 1% Total 2009 13. 0% 4. 9% 82. 0% 2014 Desai et al: JAMA 314: 2045 -53, 2015 © 2012 MFMER | slide-58
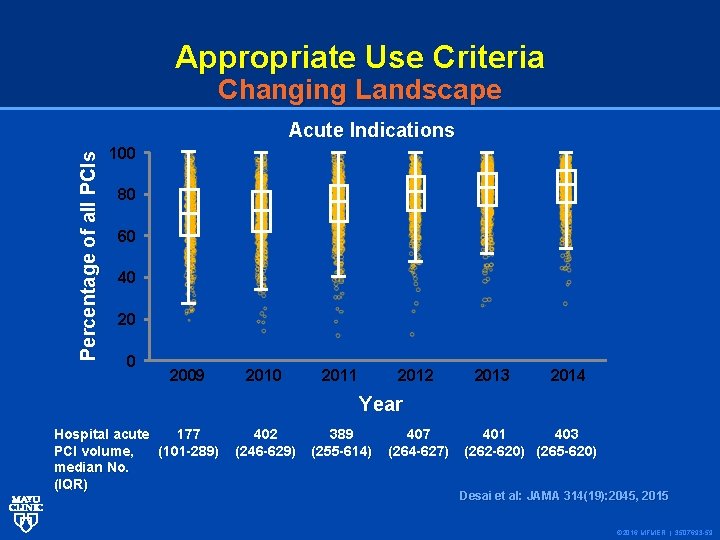
Appropriate Use Criteria Changing Landscape Percentage of all PCIs Acute Indications 100 80 60 40 2009 2010 2011 2012 2013 2014 Year Hospital acute 177 PCI volume, (101 -289) median No. (IQR) 402 (246 -629) 389 (255 -614) 407 (264 -627) 401 403 (262 -620) (265 -620) Desai et al: JAMA 314(19): 2045, 2015 © 2016 MFMER | 3507693 -59
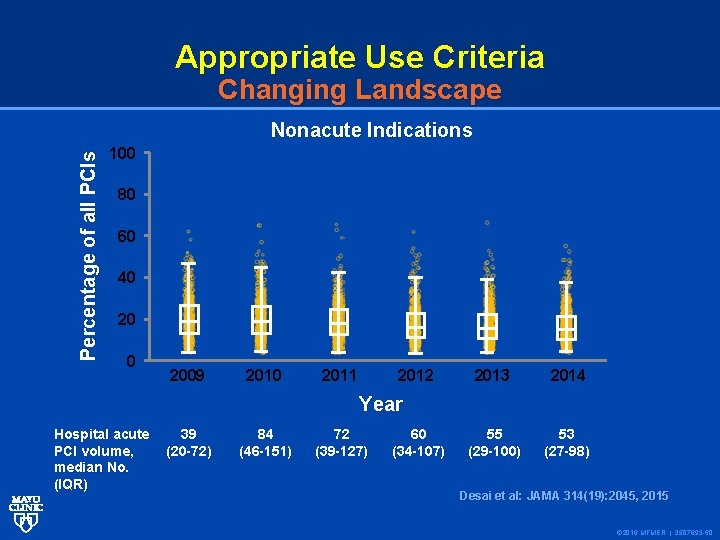
Appropriate Use Criteria Changing Landscape Percentage of all PCIs Nonacute Indications 100 80 60 40 2009 2010 2011 2012 2013 2014 55 (29 -100) 53 (27 -98) Year Hospital acute PCI volume, median No. (IQR) 39 (20 -72) 84 (46 -151) 72 (39 -127) 60 (34 -107) Desai et al: JAMA 314(19): 2045, 2015 © 2016 MFMER | 3507693 -60
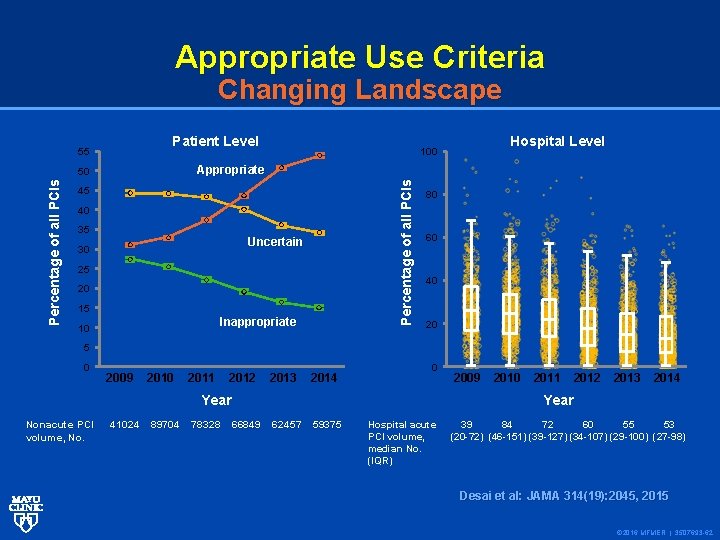
Appropriate Use Criteria Changing Landscape Patient Level 55 Percentage of all PCIs Appropriate 50 Percentage of all PCIs Hospital Level 100 45 40 35 Uncertain 30 25 20 15 Inappropriate 10 80 60 40 20 5 0 2009 2010 2011 2012 2013 2014 0 Year Nonacute PCI volume, No. 41024 89704 78328 2009 2010 2011 2012 2013 2014 Year 66849 62457 59375 Hospital acute PCI volume, median No. (IQR) 39 84 72 60 55 53 (20 -72) (46 -151) (39 -127) (34 -107) (29 -100) (27 -98) Desai et al: JAMA 314(19): 2045, 2015 © 2016 MFMER | 3507693 -62

CONCLUSIONS AND RELEVANCE Since the publication of the Appropriate Use Criteria for Coronary Revascularization in 2009, there have been significant reductions in the volume of nonacute PCI. The proportion of nonacute PCIs classified as inappropriate has declined, although hospital-level variation in inappropriate PCI persists. © 2016 MFMER | 3507693 -64
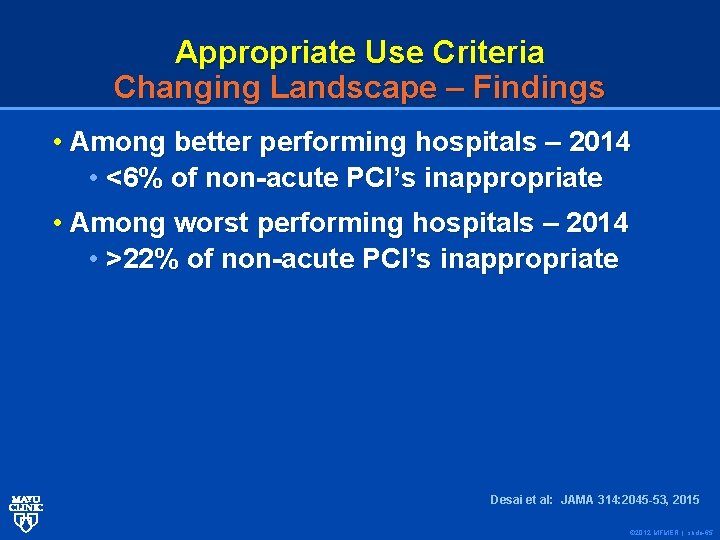
Appropriate Use Criteria Changing Landscape – Findings • Among better performing hospitals – 2014 • <6% of non-acute PCI’s inappropriate • Among worst performing hospitals – 2014 • >22% of non-acute PCI’s inappropriate Desai et al: JAMA 314: 2045 -53, 2015 © 2012 MFMER | slide-65
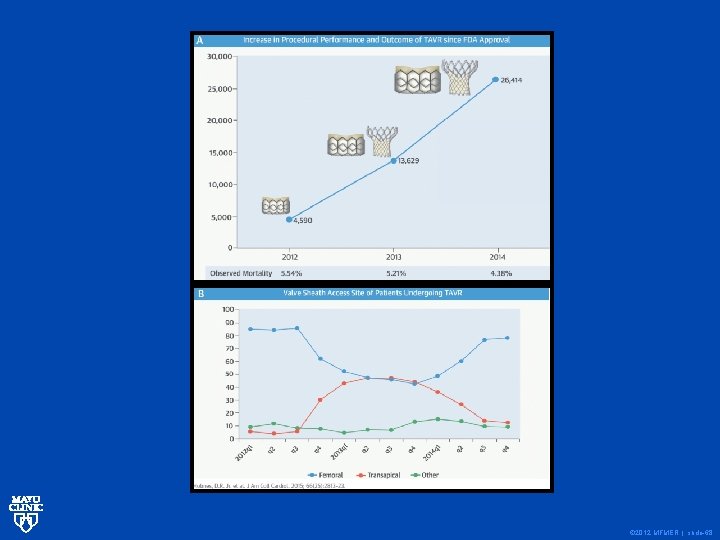
© 2012 MFMER | slide-68
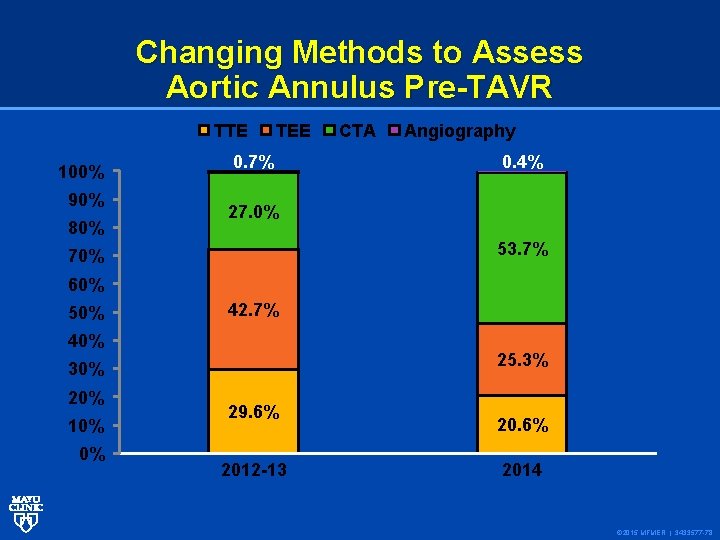
Changing Methods to Assess Aortic Annulus Pre-TAVR TTE 100% 90% 80% TEE 0. 7% CTA Angiography 0. 4% 27. 0% 53. 7% 70% 60% 50% 42. 7% 40% 25. 3% 30% 20% 10% 0% 29. 6% 2012 -13 20. 6% 2014 © 2015 MFMER | 3433577 -78
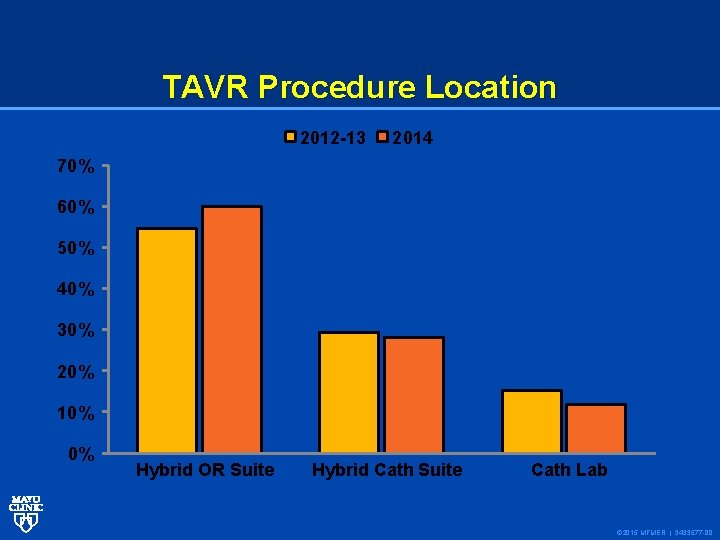
TAVR Procedure Location 2012 -13 2014 70% 60% 50% 40% 30% 20% 10% 0% Hybrid OR Suite Hybrid Cath Suite Cath Lab © 2015 MFMER | 3433577 -80
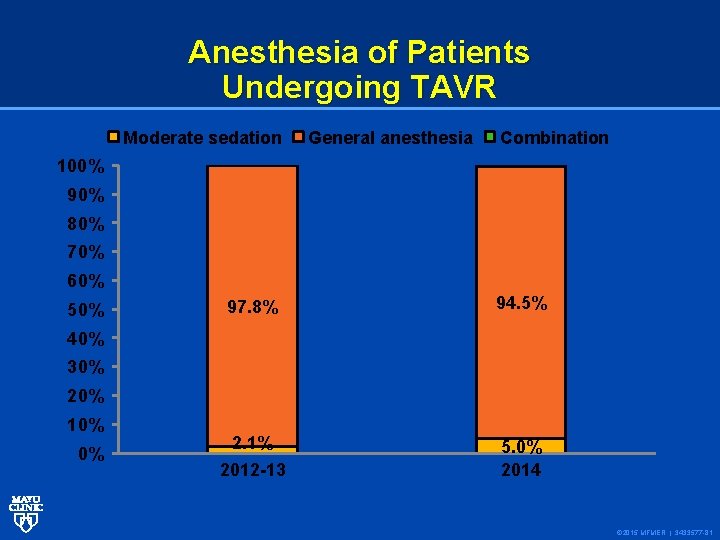
Anesthesia of Patients Undergoing TAVR Moderate sedation General anesthesia Combination 100% 90% 80% 70% 60% 50% 97. 8% 94. 5% 2. 1% 5. 0% 2014 40% 30% 20% 10% 0% 2012 -13 © 2015 MFMER | 3433577 -81
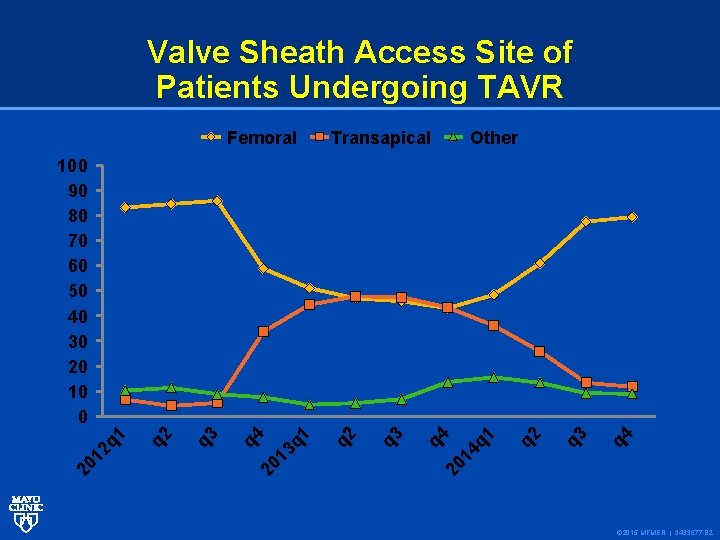
Valve Sheath Access Site of Patients Undergoing TAVR Femoral Transapical Other q 4 q 3 q 2 q 1 20 14 q 3 q 2 q 1 q 3 q 2 q 4 20 13 20 1 2 q 1 100 90 80 70 60 50 40 30 20 10 0 © 2015 MFMER | 3433577 -82
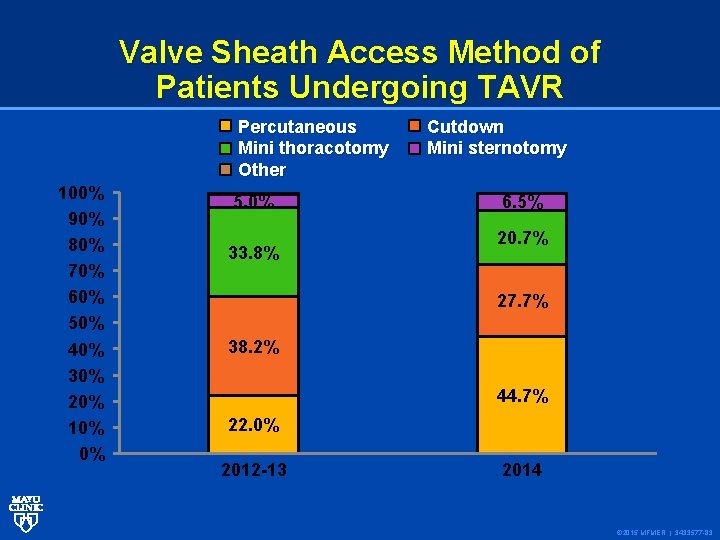
Valve Sheath Access Method of Patients Undergoing TAVR Percutaneous Mini thoracotomy Other 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% 5. 0% 33. 8% Cutdown Mini sternotomy 6. 5% 20. 7% 27. 7% 38. 2% 44. 7% 22. 0% 2012 -13 2014 © 2015 MFMER | 3433577 -83
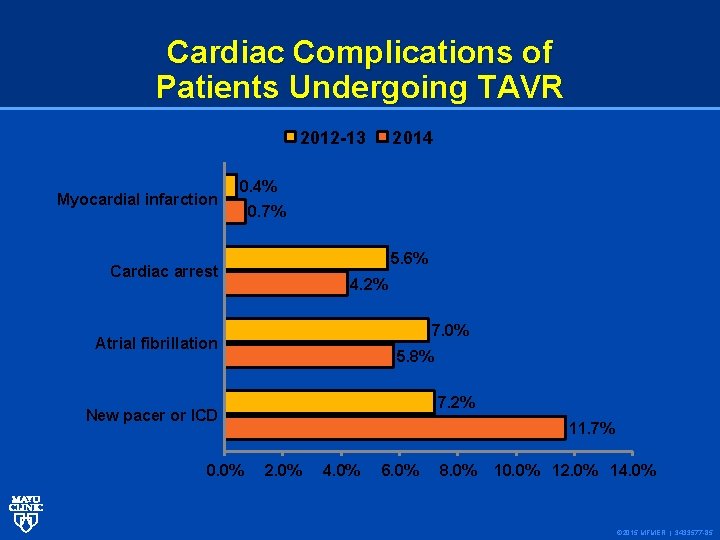
Cardiac Complications of Patients Undergoing TAVR 2012 -13 Myocardial infarction 2014 0. 4% 0. 7% 5. 6% Cardiac arrest 4. 2% 7. 0% Atrial fibrillation 5. 8% 7. 2% New pacer or ICD 0. 0% 11. 7% 2. 0% 4. 0% 6. 0% 8. 0% 10. 0% 12. 0% 14. 0% © 2015 MFMER | 3433577 -85
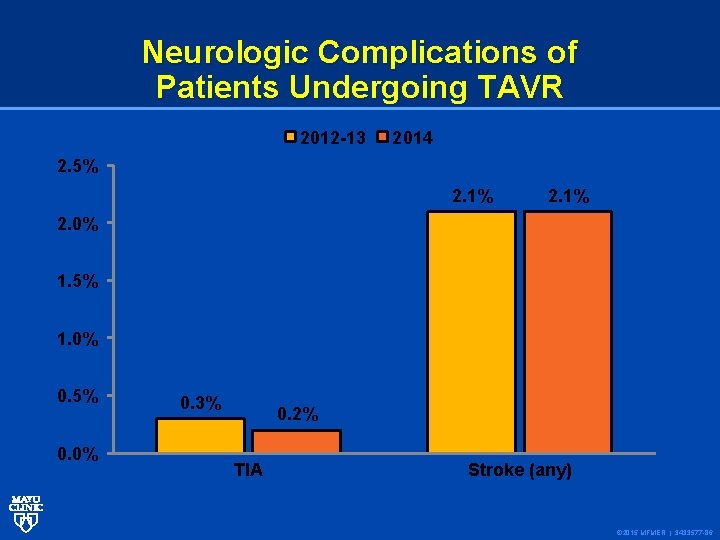
Neurologic Complications of Patients Undergoing TAVR 2012 -13 2014 2. 5% 2. 1% 2. 0% 1. 5% 1. 0% 0. 5% 0. 0% 0. 3% 0. 2% TIA Stroke (any) © 2015 MFMER | 3433577 -86
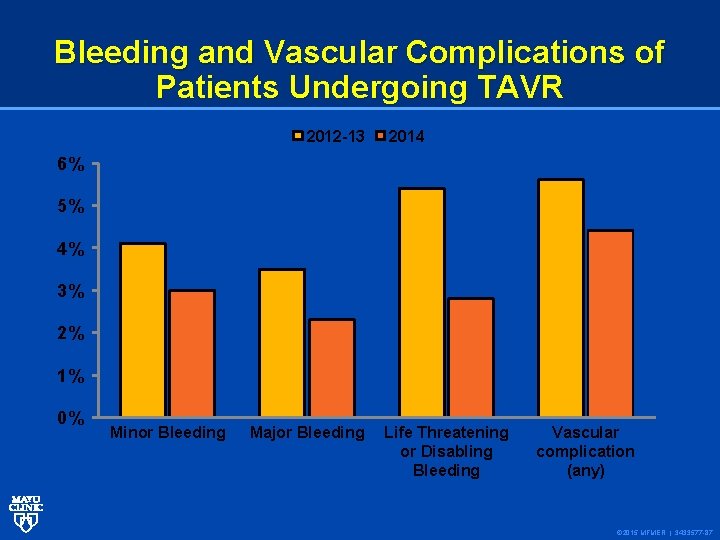
Bleeding and Vascular Complications of Patients Undergoing TAVR 2012 -13 2014 6% 5% 4% 3% 2% 1% 0% Minor Bleeding Major Bleeding Life Threatening or Disabling Bleeding Vascular complication (any) © 2015 MFMER | 3433577 -87
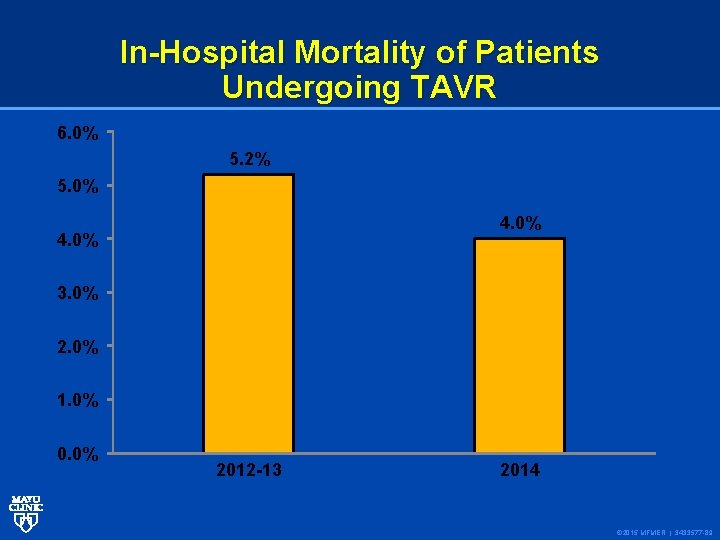
In-Hospital Mortality of Patients Undergoing TAVR 6. 0% 5. 2% 5. 0% 4. 0% 3. 0% 2. 0% 1. 0% 0. 0% 2012 -13 2014 © 2015 MFMER | 3433577 -89
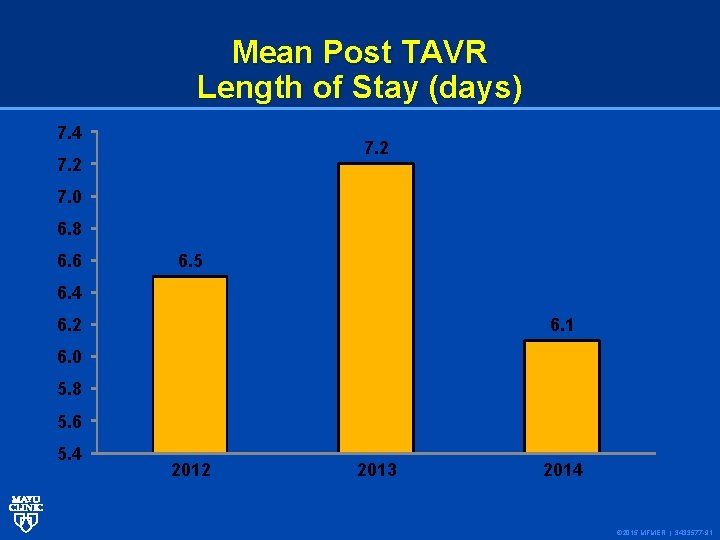
Mean Post TAVR Length of Stay (days) 7. 4 7. 2 7. 0 6. 8 6. 6 6. 5 6. 4 6. 1 6. 2 6. 0 5. 8 5. 6 5. 4 2012 2013 2014 © 2015 MFMER | 3433577 -91
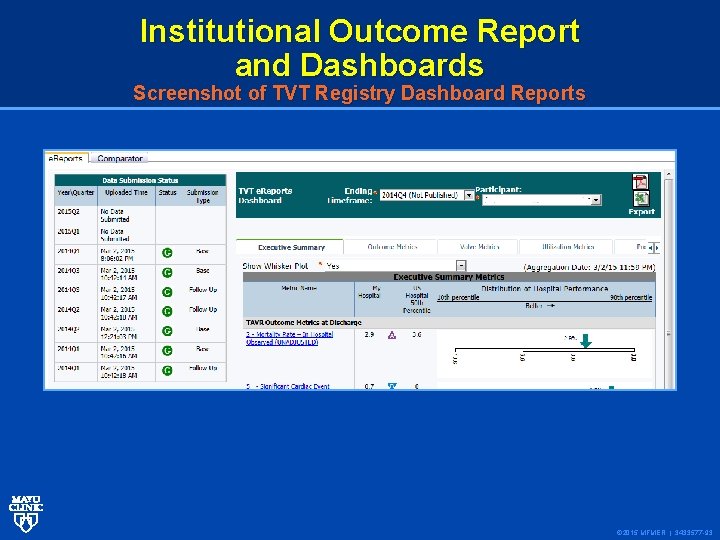
Institutional Outcome Report and Dashboards Screenshot of TVT Registry Dashboard Reports © 2015 MFMER | 3433577 -93
© 2012 MFMER | slide-98
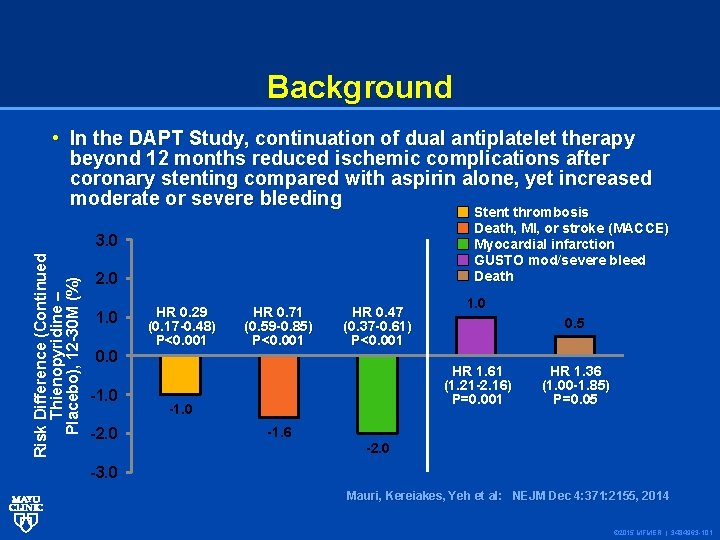
Background Risk Difference (Continued Thienopyridine – Placebo), 12 -30 M (%) • In the DAPT Study, continuation of dual antiplatelet therapy beyond 12 months reduced ischemic complications after coronary stenting compared with aspirin alone, yet increased moderate or severe bleeding Stent thrombosis Death, MI, or stroke (MACCE) Myocardial infarction GUSTO mod/severe bleed Death 3. 0 2. 0 1. 0 0. 0 -1. 0 -2. 0 HR 0. 29 (0. 17 -0. 48) P<0. 001 HR 0. 71 (0. 59 -0. 85) P<0. 001 HR 0. 47 (0. 37 -0. 61) P<0. 001 1. 0 0. 5 HR 1. 61 (1. 21 -2. 16) P=0. 001 -1. 0 -1. 6 HR 1. 36 (1. 00 -1. 85) P=0. 05 -2. 0 -3. 0 Mauri, Kereiakes, Yeh et al: NEJM Dec 4: 371: 2155, 2014 © 2015 MFMER | 3484963 -101

Objective • To develop a decision tool to identify whether an individual patient is more likely to derive benefit or harm from continuation of dual antiplatelet therapy beyond 1 year • Simultaneously accounting for risks of ischemia AND bleeding with continued therapy © 2015 MFMER | 3484963 -102
Methods – Models to Predict Ischemic and Bleeding Events Development of 2 Prediction Models within the randomized DAPT Study population (n=11, 648) • Ischemic Model: Myocardial infarction or stent thrombosis between 12 -30 months after index PCI; includes fatal events • Bleeding Model: GUSTO moderate or severe bleeding between 12 -30 months after index PCI; includes fatal events • Cox regression, stepwise selection among 37 candidate variables, including randomized treatment arm. In addition, several interaction terms with treatment arm evaluated. P value of 0. 05 for retention • Validated externally within the PROTECT trial population* *Camenzind, Wijns, Mauri et al: Lancet; 380; 9851: 1396 -1405 © 2015 MFMER | 3484963 -104
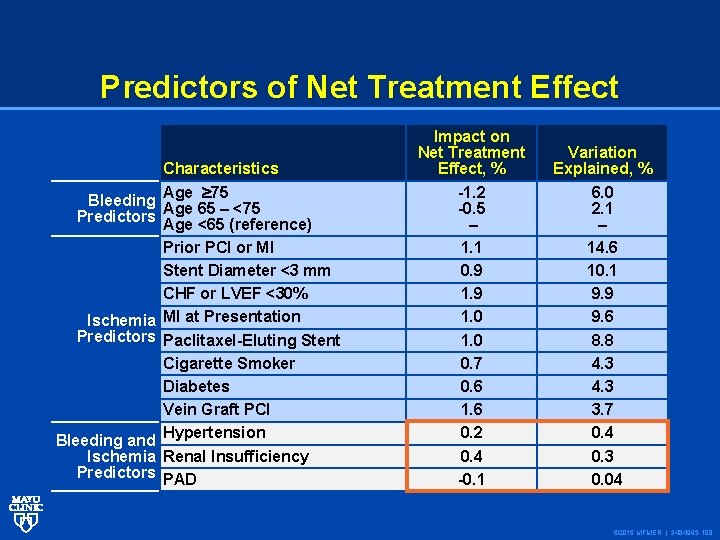
Predictors of Net Treatment Effect Characteristics Bleeding Age 75 Age 65 – <75 Predictors Age <65 (reference) Prior PCI or MI Stent Diameter <3 mm CHF or LVEF <30% Ischemia MI at Presentation Predictors Paclitaxel-Eluting Stent Cigarette Smoker Diabetes Vein Graft PCI Bleeding and Hypertension Ischemia Renal Insufficiency Predictors PAD Impact on Net Treatment Effect, % -1. 2 -0. 5 – 1. 1 0. 9 1. 0 0. 7 0. 6 1. 6 0. 2 0. 4 -0. 1 Variation Explained, % 6. 0 2. 1 – 14. 6 10. 1 9. 9 9. 6 8. 8 4. 3 3. 7 0. 4 0. 3 0. 04 © 2015 MFMER | 3484963 -108
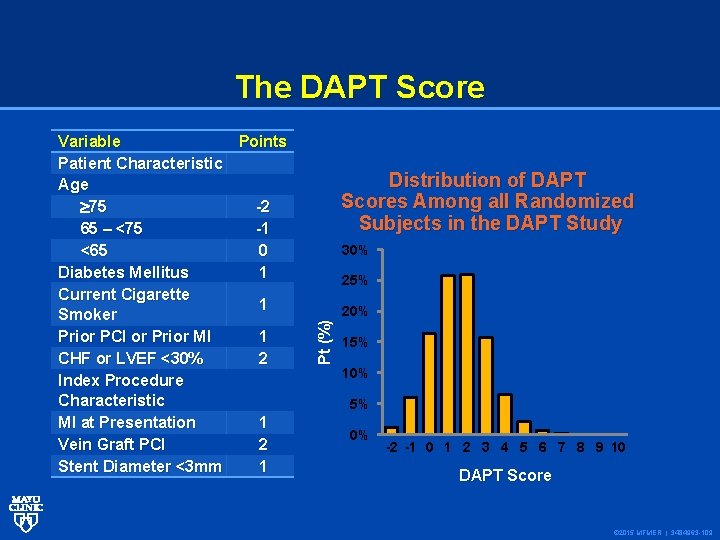
The DAPT Score Distribution of DAPT Scores Among all Randomized Subjects in the DAPT Study 30% 25% Pt (%) Variable Points Patient Characteristic Age 75 -2 65 – <75 -1 <65 0 Diabetes Mellitus 1 Current Cigarette 1 Smoker Prior PCI or Prior MI 1 CHF or LVEF <30% 2 Index Procedure Characteristic MI at Presentation 1 Vein Graft PCI 2 Stent Diameter <3 mm 1 20% 15% 10% 5% 0% -2 -1 0 1 2 3 4 5 6 7 8 9 10 DAPT Score © 2015 MFMER | 3484963 -109
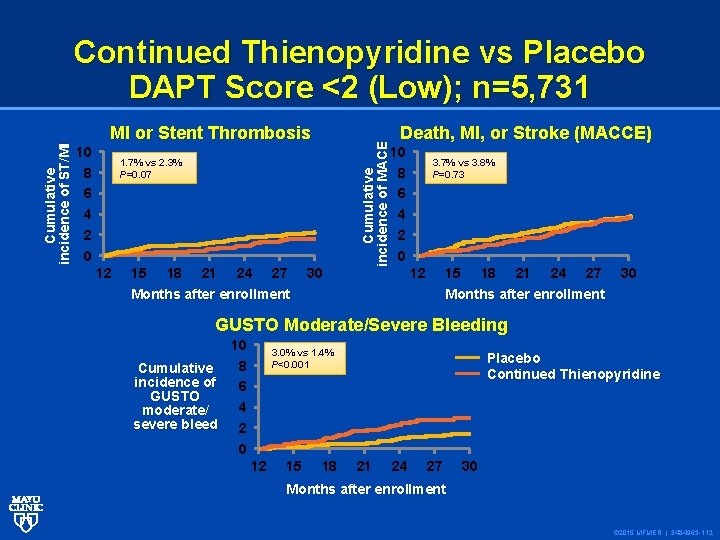
10 8 6 4 2 0 MI or Stent Thrombosis 15 18 21 24 27 Death, MI, or Stroke (MACCE) 10 8 6 4 2 0 1. 7% vs 2. 3% P=0. 07 12 Cumulative incidence of MACE Cumulative incidence of ST/MI Continued Thienopyridine vs Placebo DAPT Score <2 (Low); n=5, 731 30 3. 7% vs 3. 8% P=0. 73 12 15 Months after enrollment 18 21 24 27 30 Months after enrollment GUSTO Moderate/Severe Bleeding 10 8 Cumulative incidence of 6 GUSTO 4 moderate/ severe bleed 2 0 3. 0% vs 1. 4% P<0. 001 12 15 18 Placebo Continued Thienopyridine 21 24 27 30 Months after enrollment © 2015 MFMER | 3484963 -112
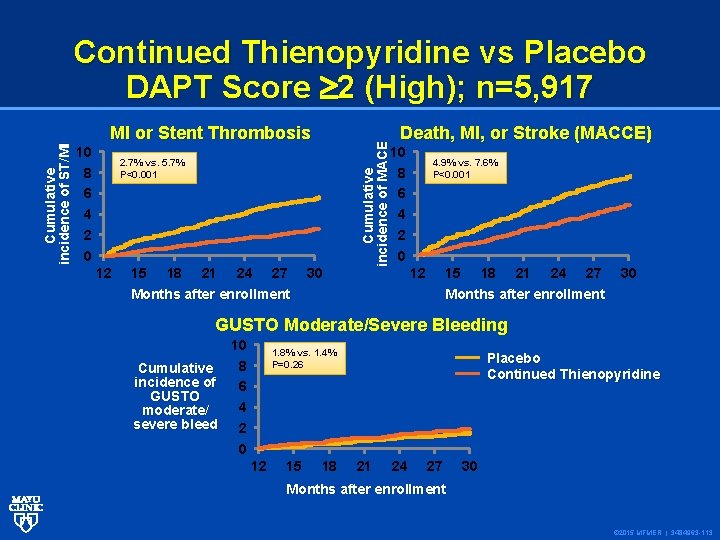
10 8 6 4 2 0 MI or Stent Thrombosis 15 18 21 24 27 Death, MI, or Stroke (MACCE) 10 8 6 4 2 0 2. 7% vs. 5. 7% P<0. 001 12 Cumulative incidence of MACE Cumulative incidence of ST/MI Continued Thienopyridine vs Placebo DAPT Score 2 (High); n=5, 917 30 4. 9% vs. 7. 6% P<0. 001 12 15 Months after enrollment 18 21 24 27 30 Months after enrollment GUSTO Moderate/Severe Bleeding 10 8 Cumulative incidence of 6 GUSTO 4 moderate/ severe bleed 2 0 1. 8% vs. 1. 4% P=0. 26 12 15 18 Placebo Continued Thienopyridine 21 24 27 30 Months after enrollment © 2015 MFMER | 3484963 -113
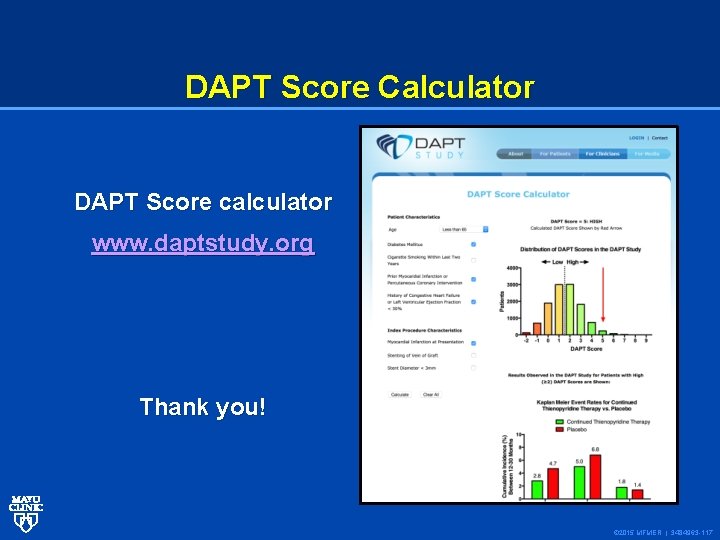
DAPT Score Calculator DAPT Score calculator www. daptstudy. org Thank you! © 2015 MFMER | 3484963 -117
Conclusions Among patients who have not had a major ischemic or bleeding event within the first year after PCI: The DAPT Score identified patients for whom ischemic benefits outweighed bleeding risks, and patients for whom bleeding risks outweighed ischemic benefits Low DAPT Score (<2) NNT to prevent ischemia = 153 NNH to cause bleeding = 64 -2 High DAPT Score 2 NNT to prevent ischemia = 34 NNH to cause bleeding = 272 10 DAPT Score may help clinicians decide who should, and who should not be treated with extended DAPT © 2015 MFMER | 3484963 -118
“Well, I’ve got your final grades ready, although I’m afraid not everyone here will be moving up. ” © 2015 MFMER | 3484963 -119

Change how we think Expand our frontiers Change what we do and how we do it Change how we respond to the environment Change how we design subsequent trials © 2012 MFMER | slide-121

© 2012 MFMER | slide-122
- Slides: 60