The 4 Most Important Elements of the Human


















- Slides: 39

The 4 Most Important Elements of the Human Body?

atom ® The smallest particle of an element that retains properties of the element ® An element has consists of only one type of atom.

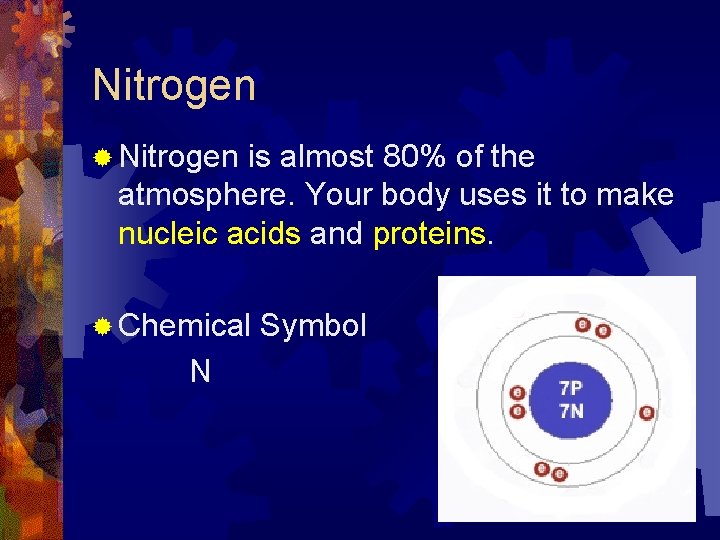
Nitrogen ® Nitrogen is almost 80% of the atmosphere. Your body uses it to make nucleic acids and proteins. ® Chemical N Symbol

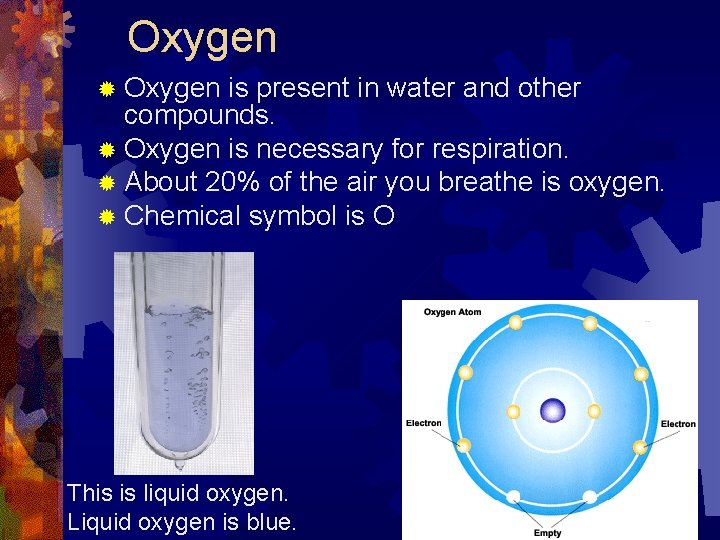
Oxygen ® Oxygen is present in water and other compounds. ® Oxygen is necessary for respiration. ® About 20% of the air you breathe is oxygen. ® Chemical symbol is O This is liquid oxygen. Liquid oxygen is blue.

Carbon ® We eat carbon and our bodies uses it for structure and our daily functions. Anything that has carbon in it is considered organic. U. S. Geological Survey Photograph of graphite, one of the forms of elemental From Chemicalelements. co

Hydrogen ® It bonds with the oxygen in our bodies to make water. H 2 O. Hubble Space Telescope, photo PR 96 -27 B NGC 604, a region of ionized hydrogen in the Triangulum Galaxy Click here to see what Steve Spangler does with oxygen and hydrogen

The 4 Most Important Elements of the Human Body? ®Just remember “NOCH” ® Nitrogen ® Oxygen ® Carbon ® Hydrogen

Elements combine to make molecules.

molecule ®A group of atoms held together by covalent bonds.

Chemical reaction ® When substances are changed by the breaking and or forming of chemical bonds.

metabolism ® All of the chemical reactions that happen within an organism

enzymes ® Act as catalysts to speed up a chemical reaction within an organism. ® Are made of proteins

homeostasis Regulation of the internal environment Of a cell or organism to maintain conditions suitable for life.

Introduction ® For each of the following you should be able to: ü Describe the properties ü List the elements found in each ü Explain the role in animals & plants • Water • Proteins • Carbohydrate s • Lipids

Water ® Water H + - O H is a polar molecule ® It forms weak hydrogen bonds ® It remains a liquid over a wide temperature range ® Water molecules stick to one another = cohesion (surface tension) ® Water molecules stick to other substances = adhesion (capillarity) +

Water ® It has a high specific heat capacity – so water can maintain a reasonably constant temperature (homeostasis) ® It has a high latent heat of vaporization – so animals use water to cool themselves ® It is less dense as a solid (ice)… ® Water is a good solvent

Lipids ® Made up of C, H and O ® Can exist as fats, oils and waxes ® They are insoluble in water ® They are a good source of energy (38 k. J/g) ® They are poor conductors of heat ® Most fats & oils are triglycerides

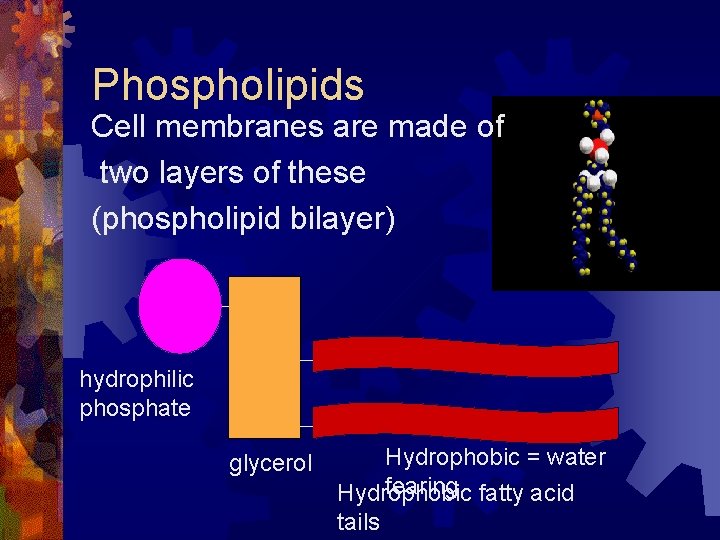
Phospholipids Cell membranes are made of two layers of these (phospholipid bilayer) hydrophilic phosphate glycerol Hydrophobic = water fearing fatty acid Hydrophobic tails

Functions of lipids ® Protection of vital organs ® To prevent evaporation in plants & animals ® To insulate the body ® They form the myelin sheath around some neurons (brain cells) (MS) ® As a component of cell membranes

Carbohydrates Contain the elements Carbon Hydrogen & Oxygen ® Includes sugars, starches and cellulose ® There are 3 types: ® Ø Ø Ø Monosaccharides Disaccharides Polysaccharides

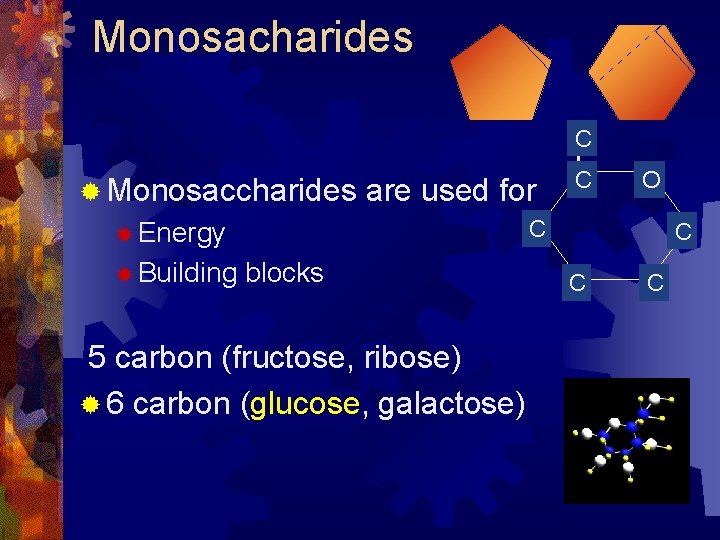
Monosacharides C ® Monosaccharides are used for O C ® Energy ® Building C blocks 5 carbon (fructose, ribose) ® 6 carbon (glucose, galactose) C C C

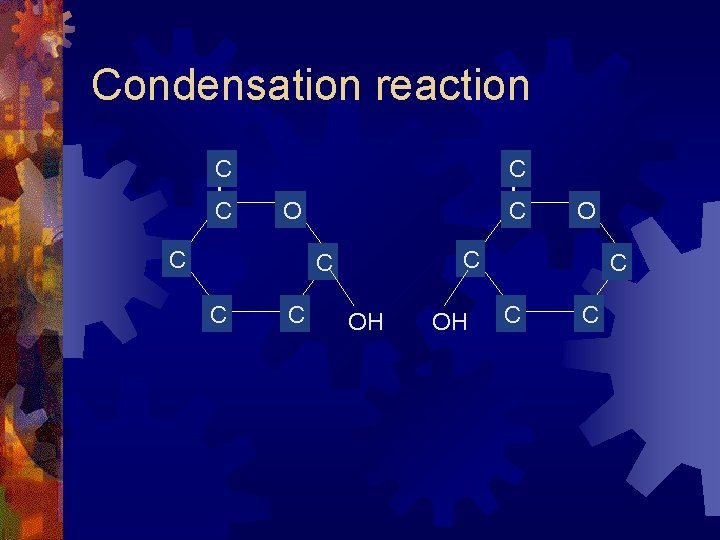
Disaccharides ® Formed from two monosaccharides ® A condensation reaction: ® (water is released) ® glucose + glucose maltose ® glucose + galactose ® glucose + fructose sucrose

Condensation reaction C C C O OH OH C C C

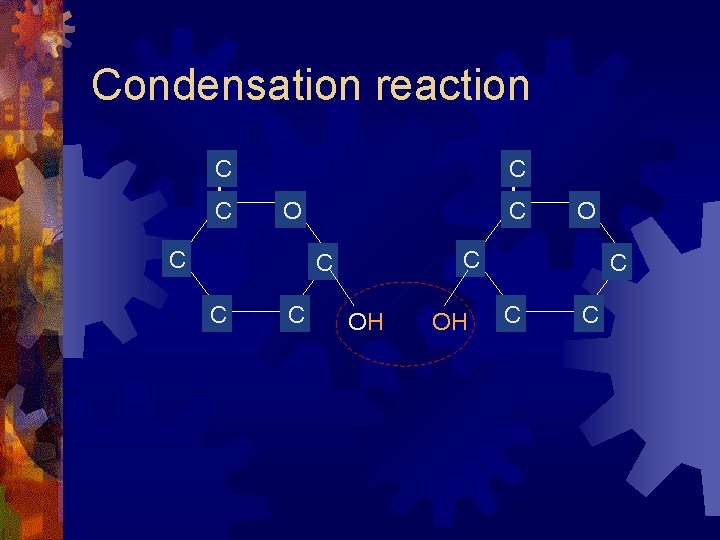
Condensation reaction C C C O OH OH C C C

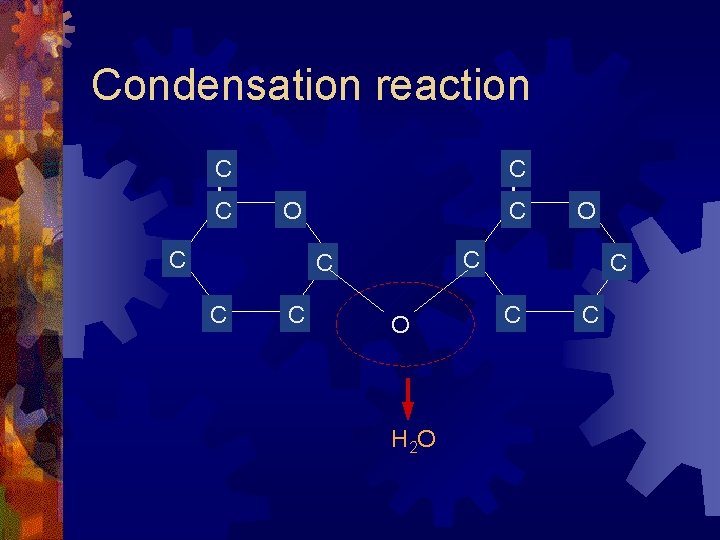
Condensation reaction C C C O O H 2 O C C C

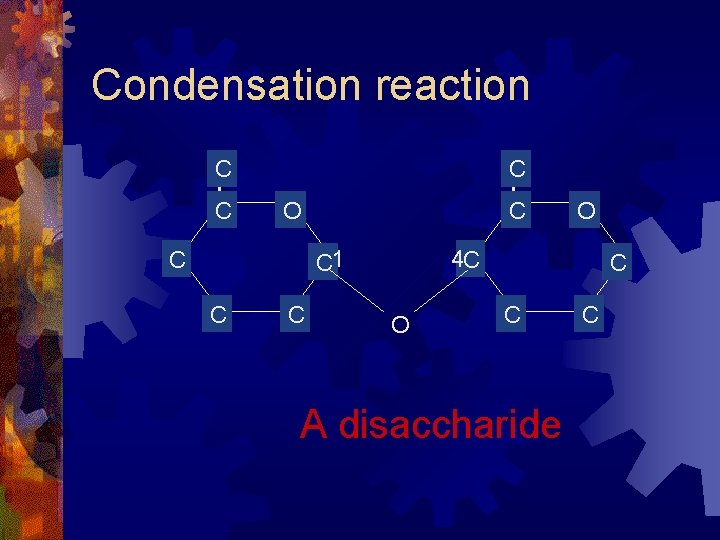
Condensation reaction C C C O C C 1 C C C O 4 C O C C A disaccharide C

Polysaccharides ® Polymers formed from many monosaccharides ® Three important examples: ® Starch ® Glycogen ® Cellulose

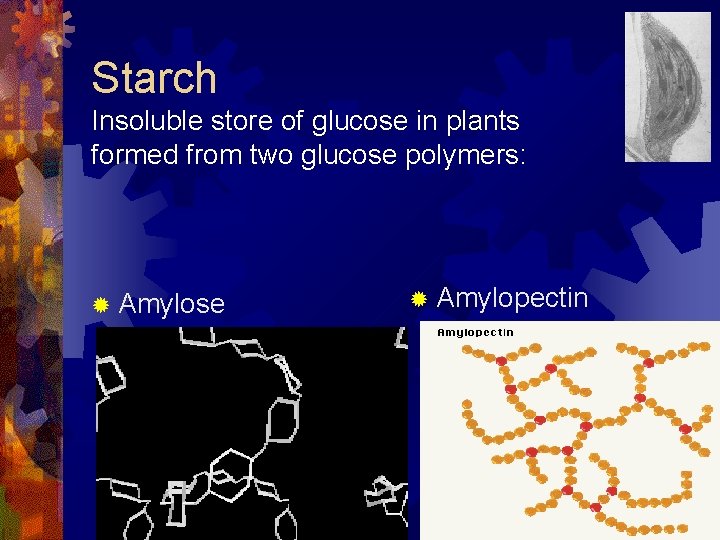
Starch Insoluble store of glucose in plants formed from two glucose polymers: ® Amylose ® Amylopectin

Glycogen ® Insoluble compact store of glucose in animals

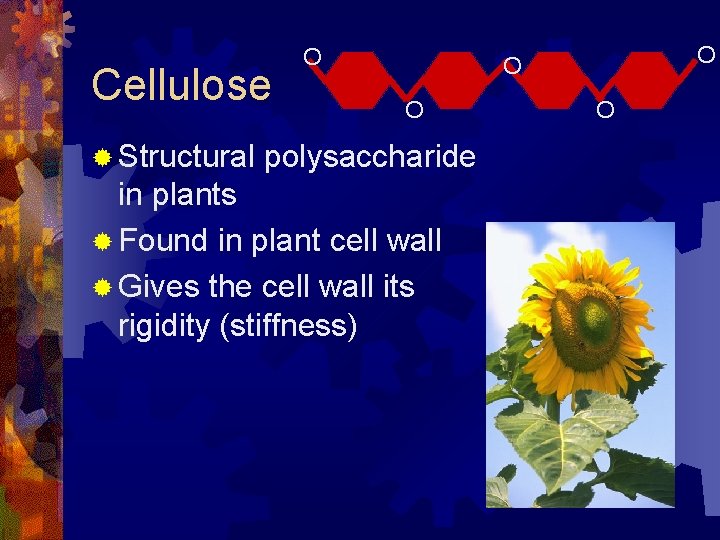
Cellulose ® Structural O O polysaccharide in plants ® Found in plant cell wall ® Gives the cell wall its rigidity (stiffness) O

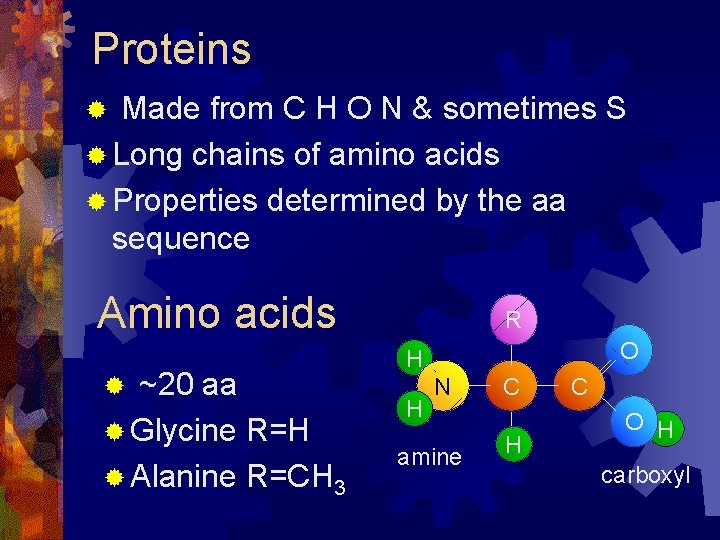
Proteins Made from C H O N & sometimes S ® Long chains of amino acids ® Properties determined by the aa sequence ® Amino acids ~20 aa ® Glycine R=H ® Alanine R=CH 3 ® R O H H N amine C H C O H carboxyl

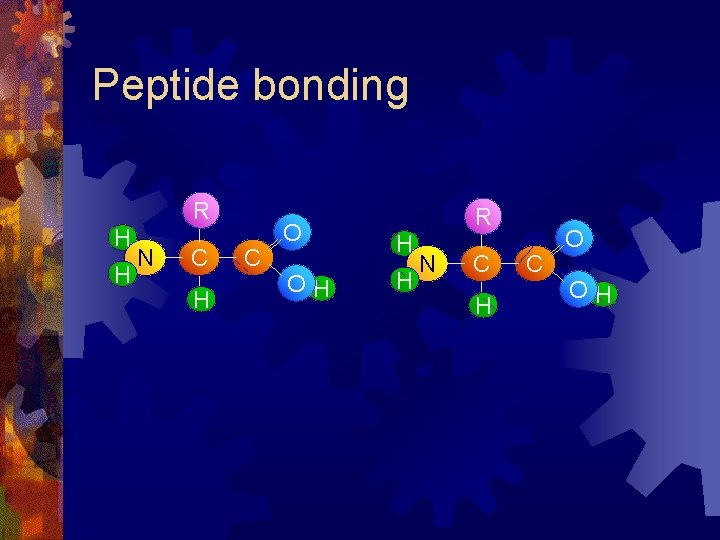
Peptide bonding R H H N C H C O OH

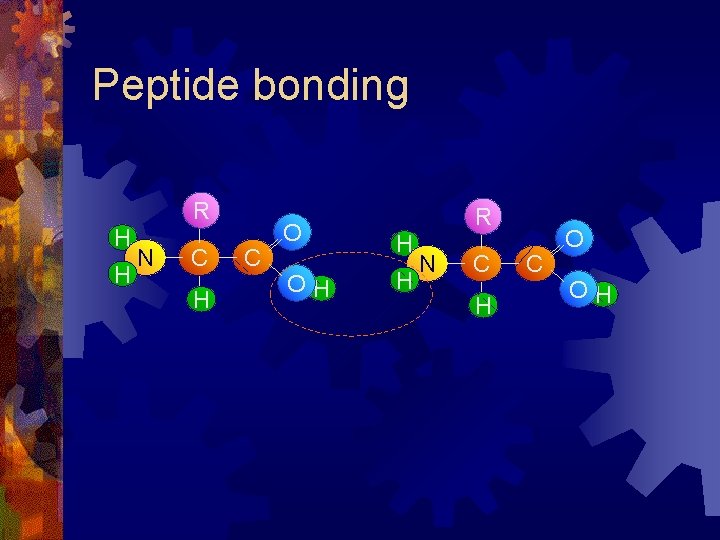
Peptide bonding R H H N C H C O OH

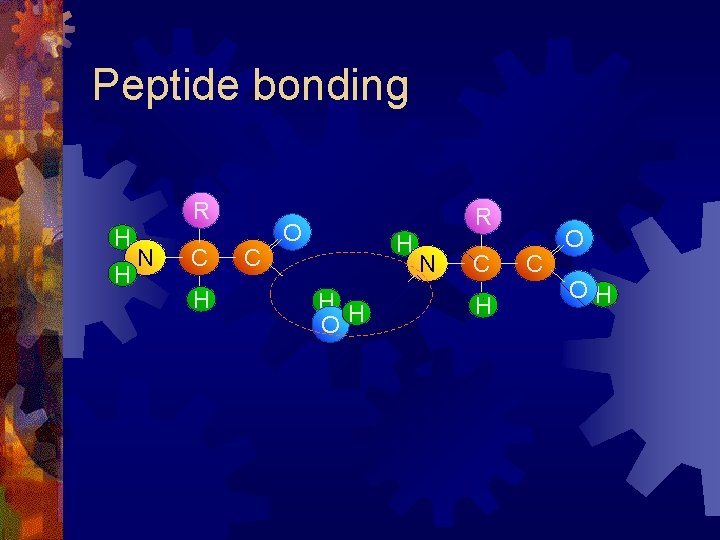
Peptide bonding R H H N C H C R O H H H O N C H C O OH

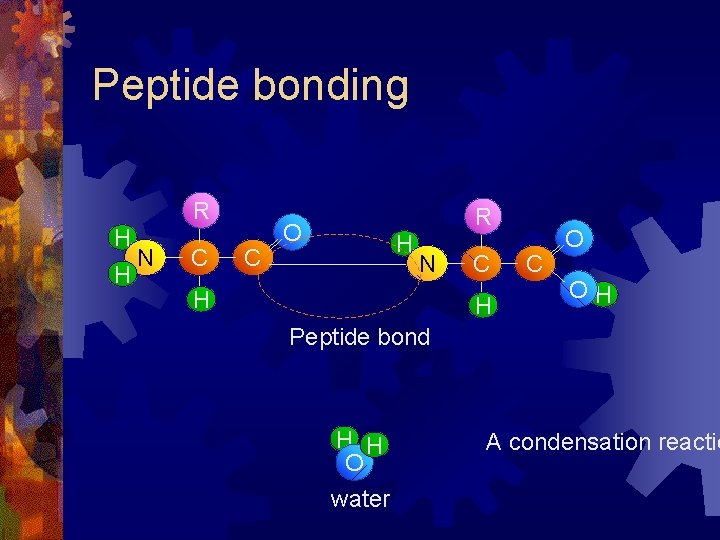
Peptide bonding R H H N C C R O H N H C O OH Peptide bond H H O water A condensation reactio

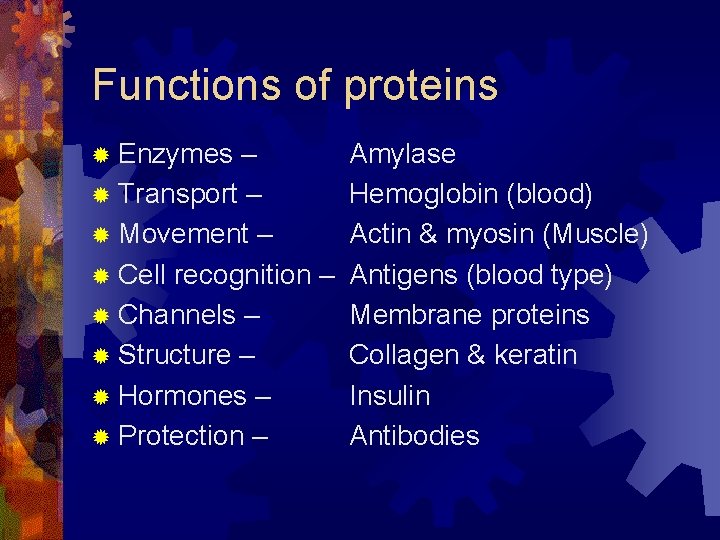
Functions of proteins ® Enzymes – ® Transport – ® Movement – ® Cell recognition – ® Channels – ® Structure – ® Hormones – ® Protection – Amylase Hemoglobin (blood) Actin & myosin (Muscle) Antigens (blood type) Membrane proteins Collagen & keratin Insulin Antibodies

Acknowledgements ® Animated cell models used by kind permission of The Virtual Cell website: ® Feel free to use this presentation for educational non-profit making purposes.

Quiz ® a) b) c) d) 1. What are important properties of water? Its polar nature Its abillity to maintain its temperature Its high latent heat of vaporization Its low density in solid form

Quiz ® a) b) c) d) 2. Sucrose is a monosacarrhide disacarrhide polysaccharide lipid