TF VIIa HEMOSTASIS TFBEARING CELL ACTIVATED PLATELET THROMBIN


TF VIIa HEMOSTASIS TF-BEARING CELL ACTIVATED PLATELET THROMBIN FIBRIN HEMOSTATIC PLUG INITIATION OF HEMOSTASIS PROPAGATION OF HEMOSTASIS
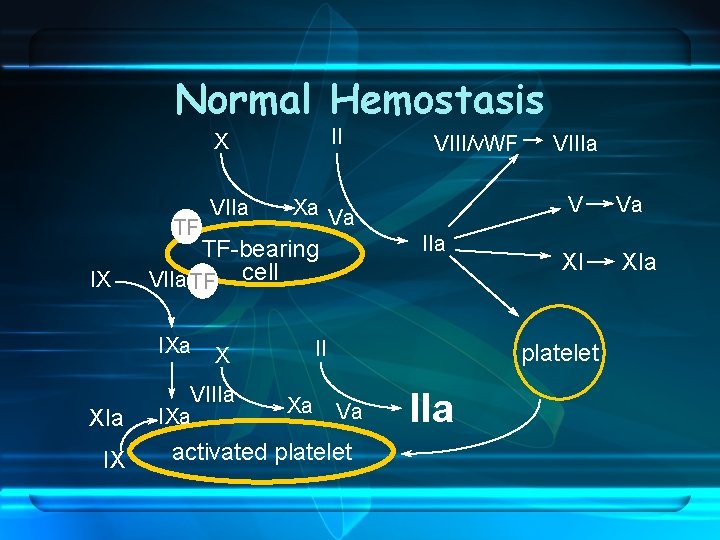
Normal Hemostasis II X TF IX IX IIa II X VIIIa IXa Xa VIIIa V Xa Va TF-bearing VIIa TF cell IXa XIa VIII/v. WF XI platelet Va activated platelet IIa Va XIa
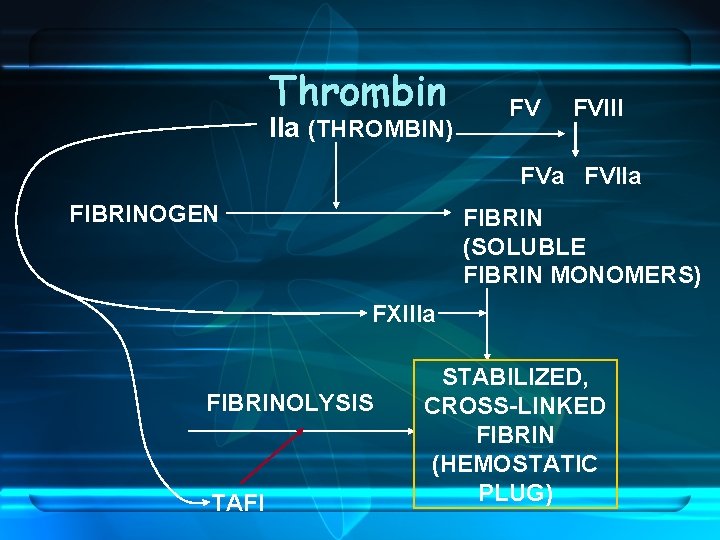
Thrombin IIa (THROMBIN) FV FVIII FVa FVIIa FIBRINOGEN FIBRIN (SOLUBLE FIBRIN MONOMERS) FXIIIa FIBRINOLYSIS TAFI STABILIZED, CROSS-LINKED FIBRIN (HEMOSTATIC PLUG)
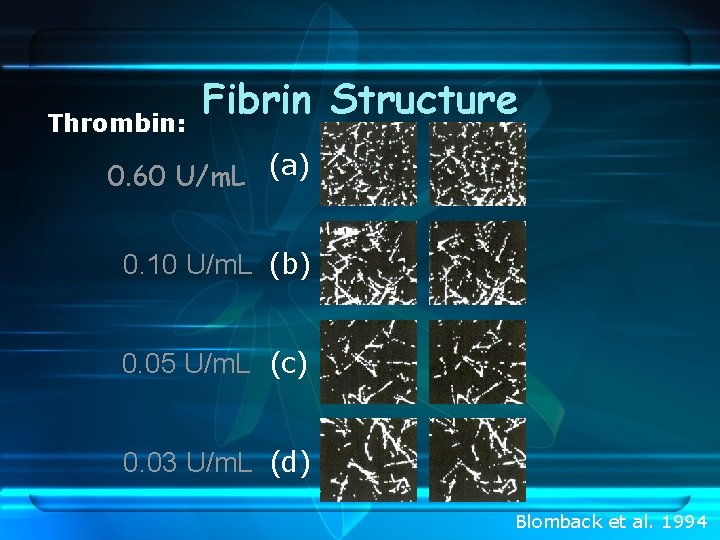
Thrombin: Fibrin Structure 0. 60 U/m. L (a) 0. 10 U/m. L (b) 0. 05 U/m. L (c) 0. 03 U/m. L (d) Blomback et al. 1994
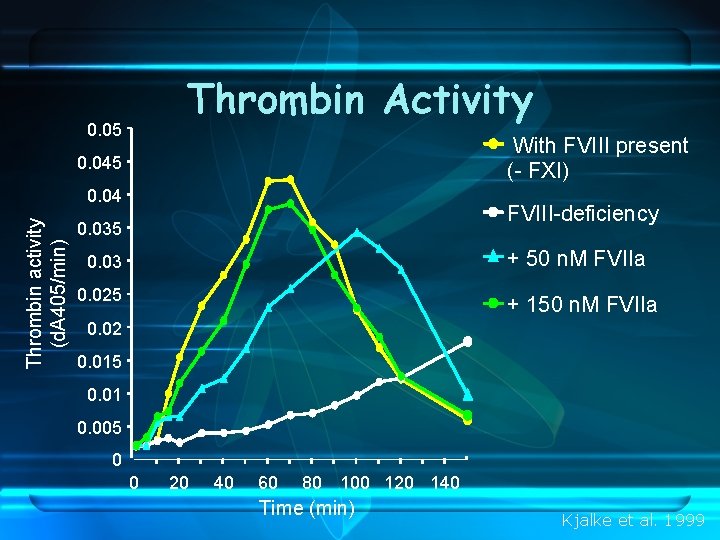
Thrombin Activity 0. 05 With FVIII present (- FXI) 0. 045 Thrombin activity (d. A 405/min) 0. 04 FVIII-deficiency 0. 035 + 50 n. M FVIIa 0. 03 0. 025 + 150 n. M FVIIa 0. 02 0. 015 0. 01 0. 005 0 0 20 40 60 80 100 120 140 Time (min) Kjalke et al. 1999
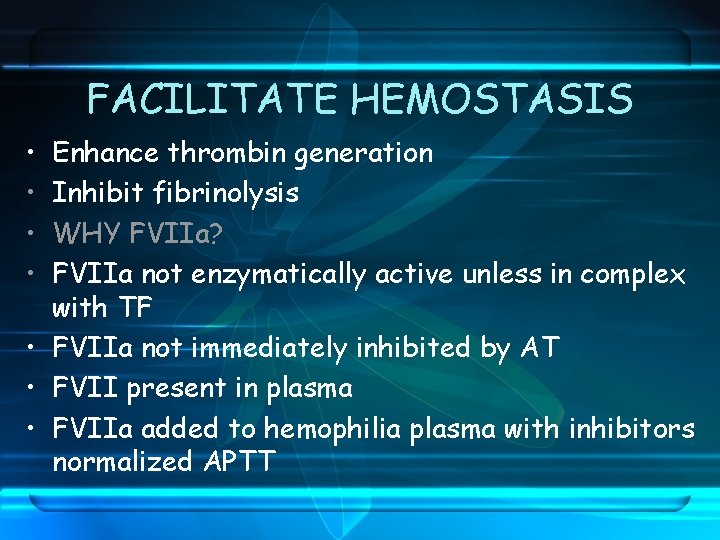
FACILITATE HEMOSTASIS • • Enhance thrombin generation Inhibit fibrinolysis WHY FVIIa? FVIIa not enzymatically active unless in complex with TF • FVIIa not immediately inhibited by AT • FVII present in plasma • FVIIa added to hemophilia plasma with inhibitors normalized APTT
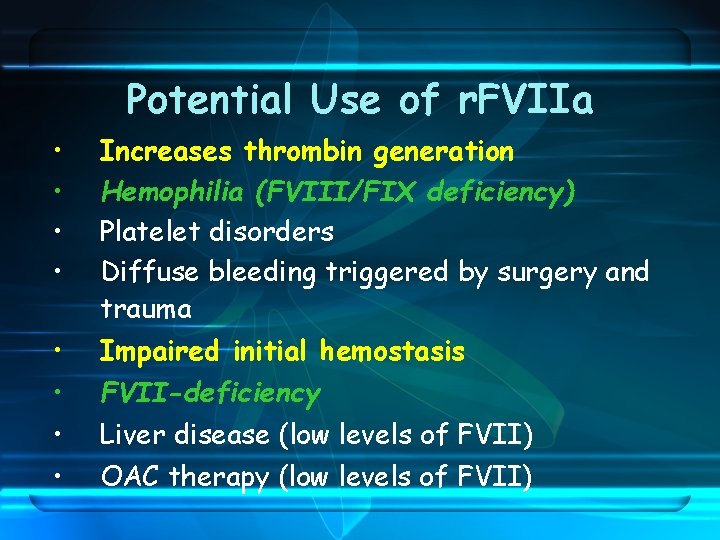
Potential Use of r. FVIIa • • Increases thrombin generation Hemophilia (FVIII/FIX deficiency) Platelet disorders Diffuse bleeding triggered by surgery and trauma Impaired initial hemostasis FVII-deficiency Liver disease (low levels of FVII) OAC therapy (low levels of FVII)
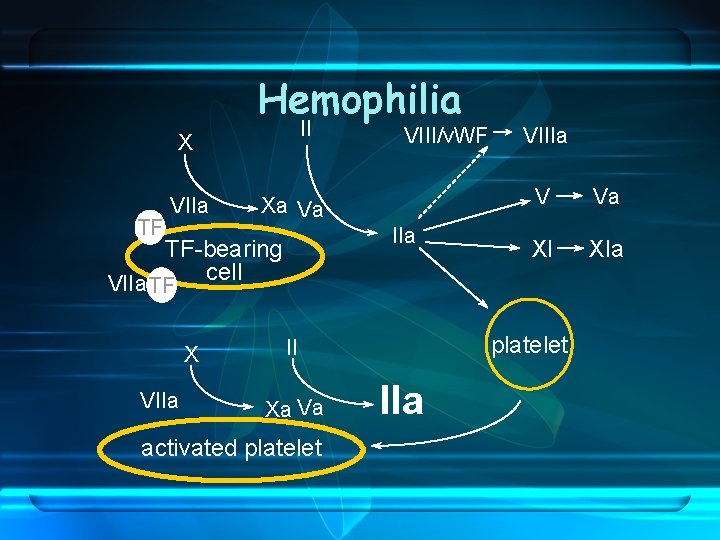
Hemophilia II X TF VIIa Xa Va IIa TF-bearing cell VIIa TF X VIIa VIII/v. WF activated platelet V Va XI XIa platelet II Xa Va VIIIa
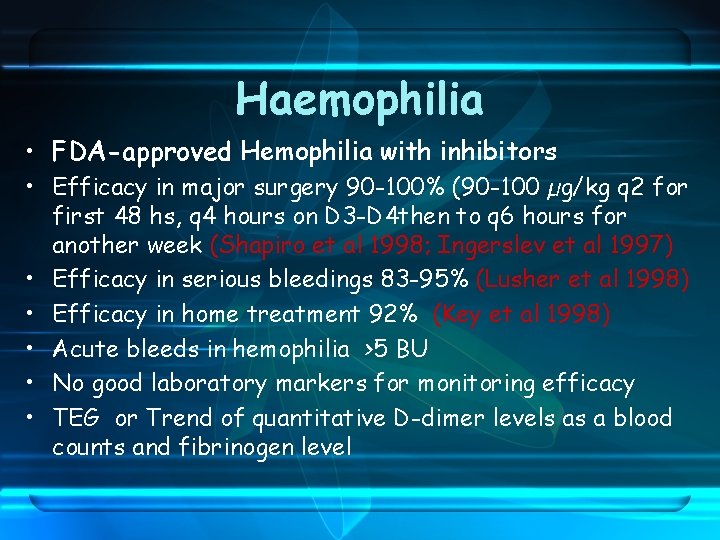
Haemophilia • FDA-approved Hemophilia with inhibitors • Efficacy in major surgery 90 -100% (90 -100 µg/kg q 2 for first 48 hs, q 4 hours on D 3 -D 4 then to q 6 hours for another week (Shapiro et al 1998; Ingerslev et al 1997) • Efficacy in serious bleedings 83 -95% (Lusher et al 1998) • Efficacy in home treatment 92% (Key et al 1998) • Acute bleeds in hemophilia >5 BU • No good laboratory markers for monitoring efficacy • TEG or Trend of quantitative D-dimer levels as a blood counts and fibrinogen level
COST-EFFECTIVENESS in mild-mod HA bleeding – UK study Clinical effectiveness – • r. FVIIa: mean 2. 3 doses of 90 ug/kg controlled 92% of all minor bleeds within 24 hrs (Key et al 1998). • FEIBA (a. PCC): mena 3 doses of 75 units/kg controlled 79% of minor bleeds within 36 hrs (Hilgartner)
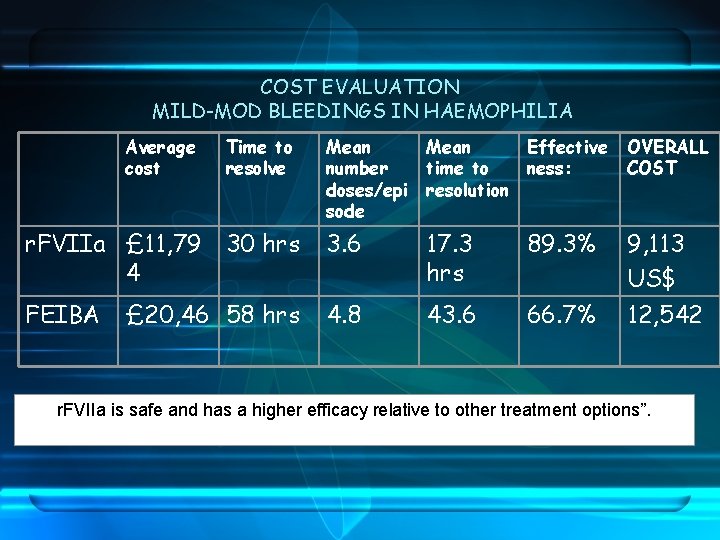
COST EVALUATION MILD-MOD BLEEDINGS IN HAEMOPHILIA Average cost Time to resolve Mean number doses/epi sode Mean Effective time to ness: resolution OVERALL COST r. FVIIa £ 11, 79 30 hrs 4 3. 6 17. 3 hrs 89. 3% 9, 113 US$ FEIBA 4. 8 43. 6 66. 7% 12, 542 £ 20, 46 58 hrs r. FVIIa is safe and has a higher efficacy relative to other treatment options”.
The FENOC study • FEIBA (activated prothrombin complex concentrate (a. PCC). • Test equivalence of products in treatment of ankle, knee, and elbow joint bleeding. • A prospective, open-label, randomized, crossover • Data for 96 bleeding episodes contributed by 48 participants were analyzed. • FEIBA and Novo. Seven appear to exhibit a similar effect on joint bleeds, although the efficacy between products is rated differently by a substantial proportion of patients. 2007 ASH
FVII-DEFICIENCY • • • Autosomal recessive 1/500 000 persons Genetic & clinical heterogeneity FVII activity <1% severe Prolonged PT; normal APTT There is only small number of patients available (case series) • FDA-approved dose 15 -30µ g per kg, q 6 -12 hs • Monitored by PT & its correction correlate well with achievement of clinical hemostasis
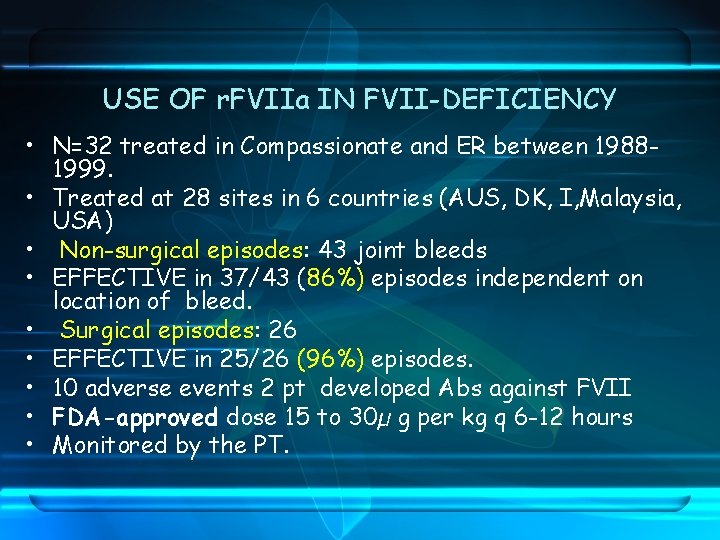
USE OF r. FVIIa IN FVII-DEFICIENCY • N=32 treated in Compassionate and ER between 19881999. • Treated at 28 sites in 6 countries (AUS, DK, I, Malaysia, USA) • Non-surgical episodes: 43 joint bleeds • EFFECTIVE in 37/43 (86%) episodes independent on location of bleed. • Surgical episodes: 26 • EFFECTIVE in 25/26 (96%) episodes. • 10 adverse events 2 pt developed Abs against FVII • FDA-approved dose 15 to 30µ g per kg q 6 -12 hours • Monitored by the PT.

• • • Preliminary guidelines for off-label use proposed in 2004 The consensus panel related use of r. FVIIa as appropriate in : Cardiac, thoracic, aortic, and spinal surgery Hepatic resection Hysterectomy; postpartum bleeding Severe, multiple trauma substantial blood replacement ineffective. Non traumatic ICH <4 hours since onset of symptoms Anti-coagulated patients with expanding hematomas. Doses of 41 to 90 µg / kg recommended in adults for all scenarios. Correction of the p. H value >7. 2 Multitrauma patients is 100 - 140 µg/ kg repeat dose
European Recommendations on the use of r. FVIIa as an adjunctive treatment for massive bleeding –

Trauma. • Uncontrolled massive hemorrhage is 2 nd cause of death • Massive hemorrhage : surgical / vascular and a coagulopathic component. • ‘Lethal triad‘: consumption , dilution and metabolic disorders • In cases of injury, TF is brought into contact with naturally occurring FVIIa, to initiate thrombin • Pharmacological doses, r. FVIIa bind activated platelets at the site of injury and activate FIX and X directly, leading to a thrombin burst

• • • Blunt. Trauma Successful report for trauma in an Israeli soldier Case series of 36 patients stopped bleeding in 72% of cases Several case studies , case series , retrospective cohort Conventional hemostatic measures have failed. Promising addition to thrapeutic armamentarium Multicenter, randomized, double-blind, placebo controlled study by Boffard • Initial dose of 200 µg/kg , then 100 µg/kg, at 1 and 3 hours • Produced a significant reduction in the primary endpoint RBC transfusion requirements , need for massive transfusion, and incidence of respiratory failure Grade B • Penetrating trauma are uncertain, no recommendations can be made for this indication. Grade B
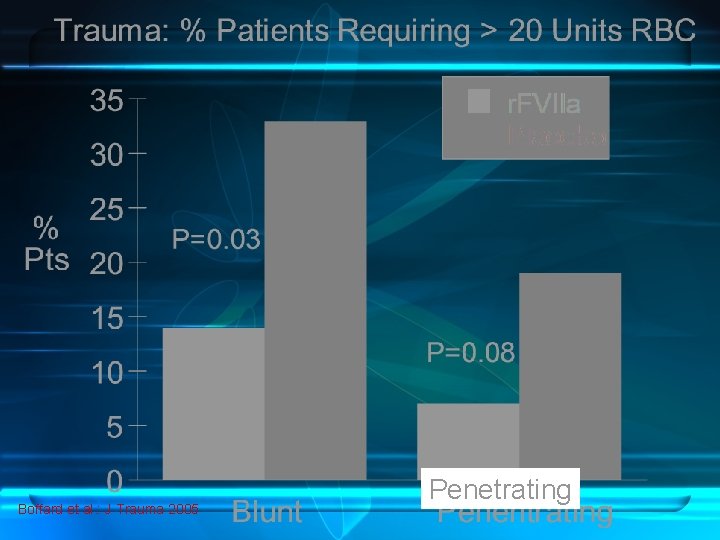
Boffard et al. : J Trauma 2005 Penetrating
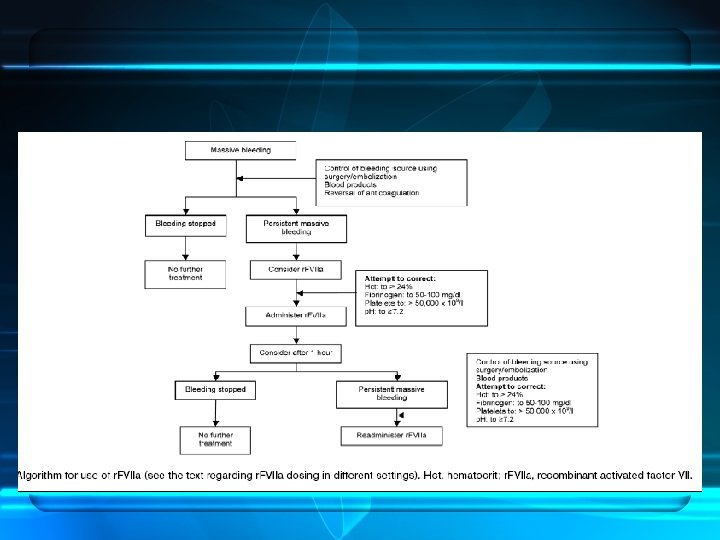

Recommendations on the use of r. FVIIa as an adjunctive treatment for massive bleeding – a European perspective • • • Not be used in prophylactically in elective surgery (grade A) Use of r. FVIIa in blunt trauma (grade B). Not be recommended for use in penetrating trauma (grade B) Not be recommended for use in liver surgery (grade B) Not be recommended for use in or in bleeding episodes in patients with Child–Pugh A cirrhosis (grade B). Bleeding after cardiac surgery (grade D). Postpartum hemorrhage (grade E) Uncontrolled bleeding in surgical patients (grade E) Monitoring of r. FVIIa efficacy should be performed visually and by assessment of transfusion requirements (grade E), Critical Care 2006, 10: R 120

Warfarin Reversal • • Dramatic increase in number of patients receiving OAC Interindividual variation (environmental and genetic) Incidence of fatal haemorrhage : 1%/Y. Increased risk of ICH > 50 y compared with nonanticoagulated 10 x • Reversal : seriousness of bleeding , thrombotic risk and speed and completeness of reversal • Options : dose omission , vit K & factors replacement • FFP or PCCs

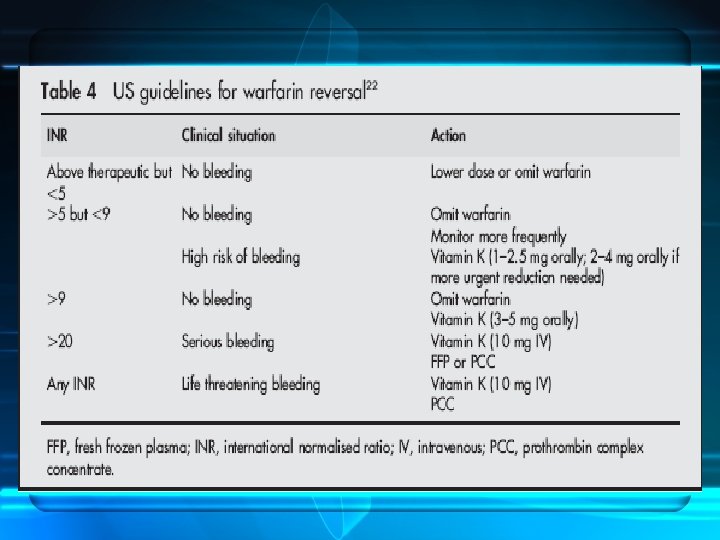

• • Warfarin Reversal PCCs Intermediate purity plasma products Only HTDEFIX is licensed in UK for warfarin reversal PCCs, (‘‘ 4 factor concentrates’’), OR low VII (3 ) Amounts of protein C and S Optimum dose not established. Thrombogenicity, exacerbation of DIC are dose related Current cost in UK (single treatment for a 70 kg individual £ 437 -£ 875). • More expensive > FFP. ( unit of produced from UK plasma costs about £ 30). • FFP that is methylene blue treated or produced from non -UK plasma is more expensive. )

• • • Warfarin Reversal r. FVIIa Advocated in the management of bleeding Studied in a small number of studies Normalises the INR in anticoagulated Dose range, 15– 90 mg/kg Small numbers of patients with ICH successfully treated with r. FVIIa have been reported recently. 54– 56 • Lin J, J Neurosurg 2003; 98: 737– 40. • Sorensen B, Blood Coagul Fibrinolysis 2003; 14: 469– 77 • On the basis of this limited data, the role of r. FVIIa in warfarin reversal remains unclear.

Use in intracranial hemorrhage. • A recent report in ICH in adults • Control the expansion of intracranial hematomas in elderly patients, improving neurologic outcome and significantly decreasing mortality. • Serious thromboembolic events were higher in the treated groups (7% vs. 2% for placebo). • Bijsterveld NR, Circulation 2002; 106: 2550 -4.
SAFETY PROFILE • Theoretical increased risk of thrombotic events • r. FVIIa bind to active PLTs , hemostatic activity should be restricted to vessel injury (TF is exposed & PLTs are locally activated) • Experimental evidence for localized effect in rabbit model • Dec 1995 -Jan 2005, total amount of r. FVIIa released 680, 245 standard doses : approved or “off-label” use, • Over this postmarketing period, 123 thrombotic corresponding to a mean of 1/10000 thrombotic • Review in patients with acquired and congenital hemophilia with inhibitors, incidence of thrombotic events was low

SAFETY PROFILE • Review of 13 controlled clinical trials, 1178 patients with coagulopathy No significant association was found between exposure to r. FVIIa and incidence of thrombotic events? ? . • No inhibitors reported neither in HA nor off-label use. • Two patients with FVII-DEFICIENCY (no FVII protein) developed transient inhibitors against FVII. • Thrombotic complication: elderly with existing atherosclerotic disease. • FDA report : Arterial and venous thromboembolic events. Half occurred in first 24 hours after last r. FVIIa dose. • Underlying medical conditions existed in some. • Lack sufficient information dosage , concomitant medications, pre-existing medical conditions and the confounding indication;

Advantages Disadvantages Advantages • Rapid onset of action • Low-volume dosing • Recombinant nature alleviating infectious disease transmission • Low risk of thrombogenicity : increasing cases being reported of thromboembolic manifestations Disadvantages • Substantial cost $1000 per milligram • Risk of thrombosis • Variability of current recommended dose and dosing intervals • Short half-life • Limited data pertaining to safety and efficacy, • Problems with monitoring its efficacy.

SUMMARY • Great potential in achieving hemostasis in patients refractory to traditional treatments. • Significant cost and uncertain benefit in many clinical situations, it should not be used indiscriminately. • Transfusion service , pharmacy OR content expert in hemostasis are appropriate gatekeepers • The ordering physician must demonstrate to a gatekeeper that the patient meets established criteria • For off-label use a maximum of two doses • Further doses given only after additional expert consultation. • FDA-approved indication : Hemophilia patients with inhibitors & Congenital FVII deficiency

Thanks
Hemostatic Defects Most common are: - Low platelet counts - Low levels of vit K-depandent coagulation factors (FVII, FX, FII, Prot. C) - lowered fibrinogen - lowered FVIII and FV - increased fibrinolysis
Use in qualitative PLT disorders. • Ability of pharmacologic doses to enhance rate of thrombin generation on activated PLTs • Midlevel evidence and case reports exist. • Glanzmann’s thrombasthenia : reports with good results. • A report of 33 episodes in 7 children 60% excellent response if treated within 12 hours of onset of bleeding. • Surgical prophylaxis and excessive menstrual bleeding • Doses of 90 to 120µ g per kg • Approved for use in Europe • Poon MC, international survey. J Thromb Haemost 2004; 2: 1096 -103.
- Slides: 35