Tetrahedral Crystal Field Splitting Why do dorbitals split

Tetrahedral Crystal Field Splitting Why do d-orbitals split in these peculiar ways in presence of Tetrahedral ligand fields? This interactive instruction animation will explain things for the beginner student of Inorganic Chemistry Dhruv Joshi Department of Chemistry IIT Bombay

INSTRUCTIONS TO ANIMATOR In this IDD, first the master layout and the definitions of components will be shown. After that the slides will be shown in the order which the user will be seeing them. Slides will initially contain some theory and then will have a simple interactive animation. Please follow the order shown, which will help the user understand the concept very well.
1 Master Layout 2 WORKSPAC 3 4 5 TEXT

1 2 3 Definitions of the components: 1. orbital: These are the regions in an atom where electrons are most likely to be found 2. d-orbitals: These are a certain set of orbitals which are found filled in transition metals like Iron, Copper etc. They are important in the study of Complex compounds, which this IDD is dealing with. The d-orbitals are of two types: t 2 g and eg 3. Complex compounds: compounds made most commonly by Transition metals (like iron, copper, nickel) which involve special bonds, and hence these are classified seperately as complex””. 4. Ligands: These are negatively charged compounds which attach to a transition metal to make complex compounds 4 5

1 2 3 4 5 Analogy / Scenario / Action In forming a complex compound, the d-orbitals of a transition metal undergo some changes which cause the complex compounds to have the unique properties which they do. The so-called “Crystal Field Theory” explains this by electronic repulsion between ligands and the electrons in d-orbitals. The ligands approach the metal from different directions, and depending on which orbital is closest to their direction of approach, they cause the energy of it to increase, due to electronic repulsions.
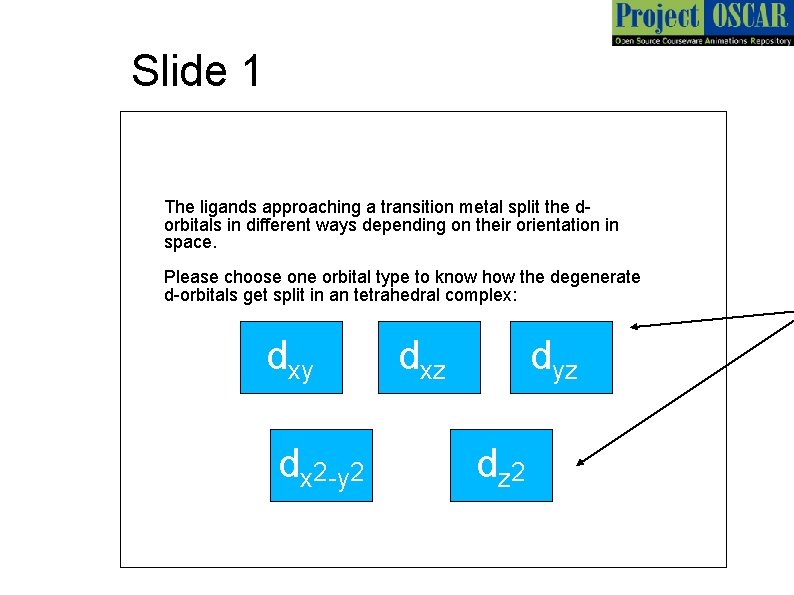
Slide 1 The ligands approaching a transition metal split the dorbitals in different ways depending on their orientation in space. Please choose one orbital type to know how the degenerate d-orbitals get split in an tetrahedral complex: dxy dx 2 -y 2 dxz dyz dz 2
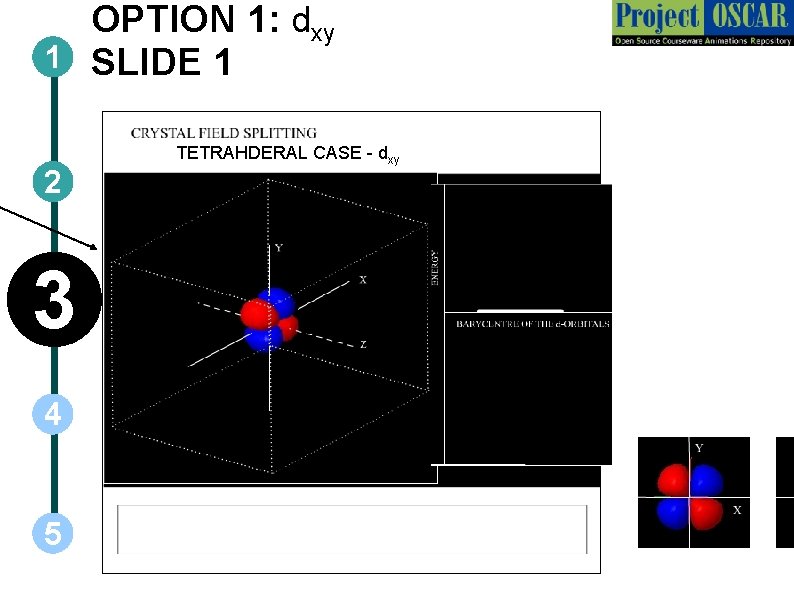
OPTION 1: dxy 1 SLIDE 1 2 3 4 5 TETRAHDERAL CASE - dxy
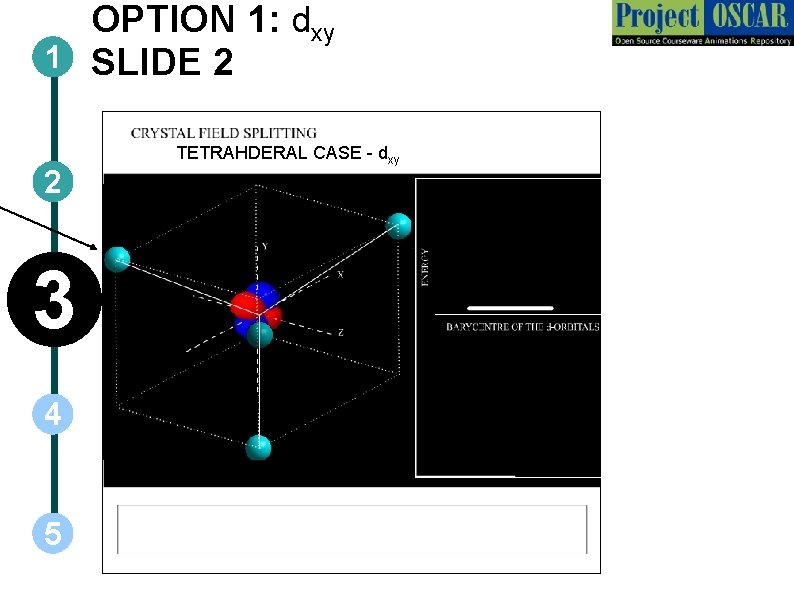
OPTION 1: dxy 1 SLIDE 2 2 3 4 5 TETRAHDERAL CASE - dxy
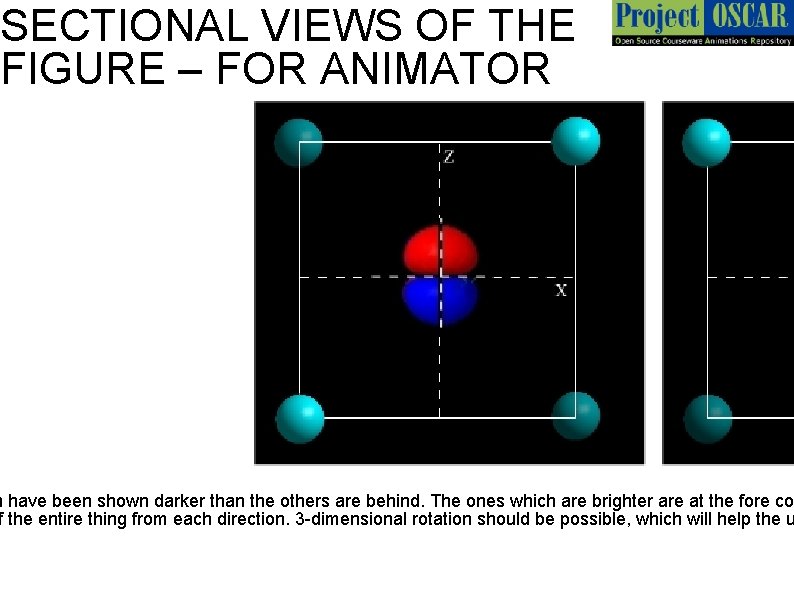
SECTIONAL VIEWS OF THE FIGURE – FOR ANIMATOR h have been shown darker than the others are behind. The ones which are brighter are at the fore co f the entire thing from each direction. 3 -dimensional rotation should be possible, which will help the u
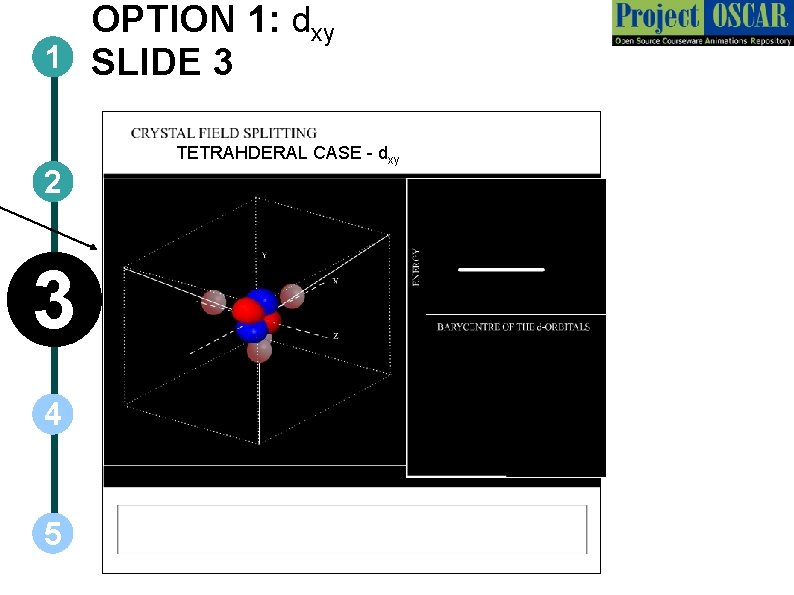
OPTION 1: dxy 1 SLIDE 3 2 3 4 5 TETRAHDERAL CASE - dxy
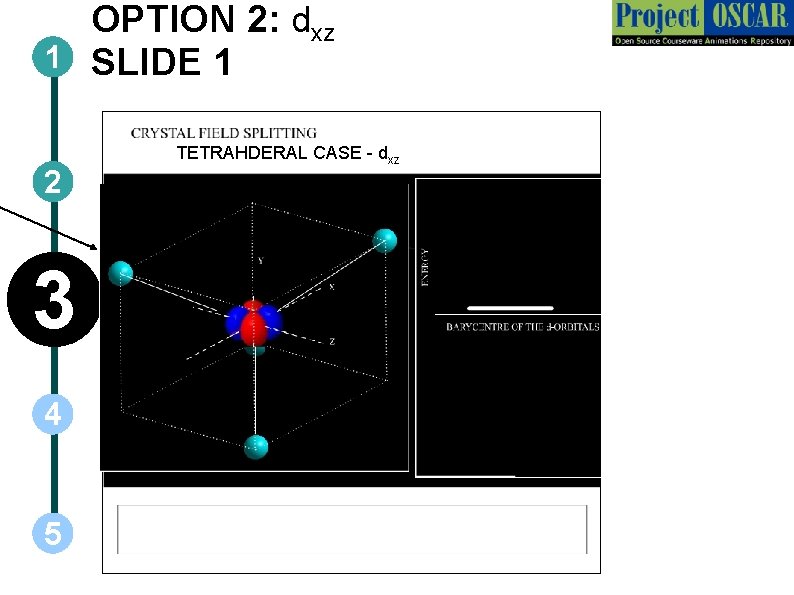
OPTION 2: dxz 1 SLIDE 1 2 3 4 5 TETRAHDERAL CASE - dxz
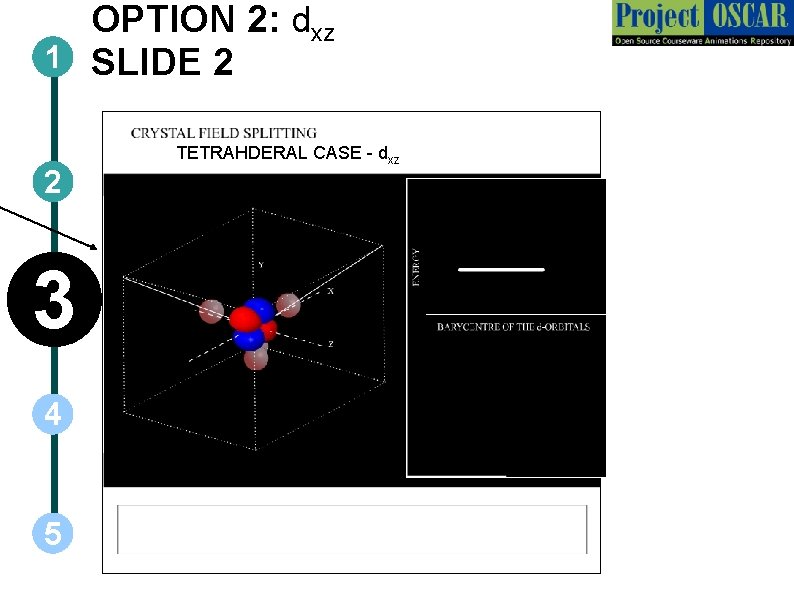
OPTION 2: dxz 1 SLIDE 2 2 3 4 5 TETRAHDERAL CASE - dxz
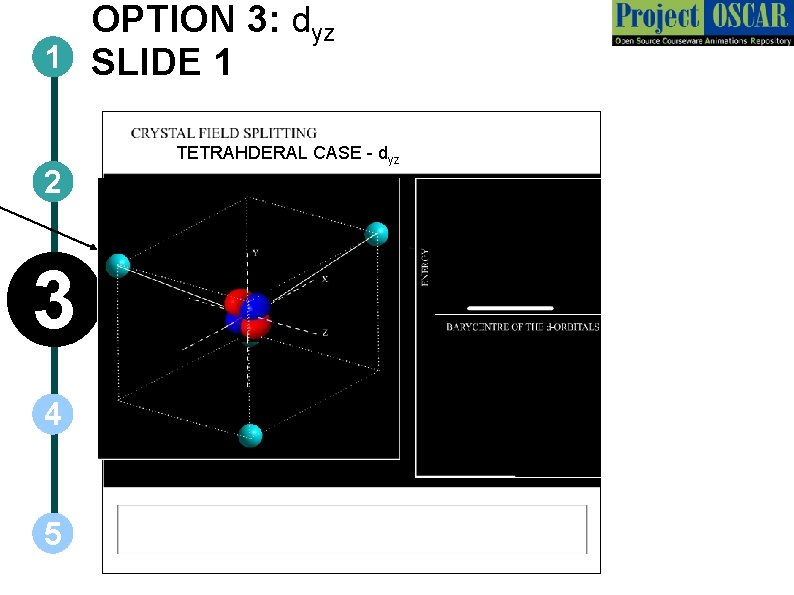
OPTION 3: dyz 1 SLIDE 1 2 3 4 5 TETRAHDERAL CASE - dyz
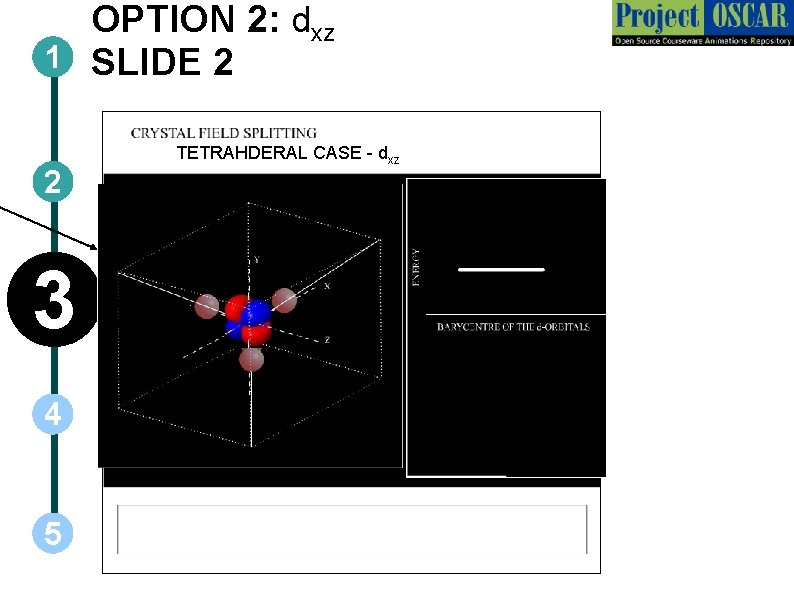
OPTION 2: dxz 1 SLIDE 2 2 3 4 5 TETRAHDERAL CASE - dxz
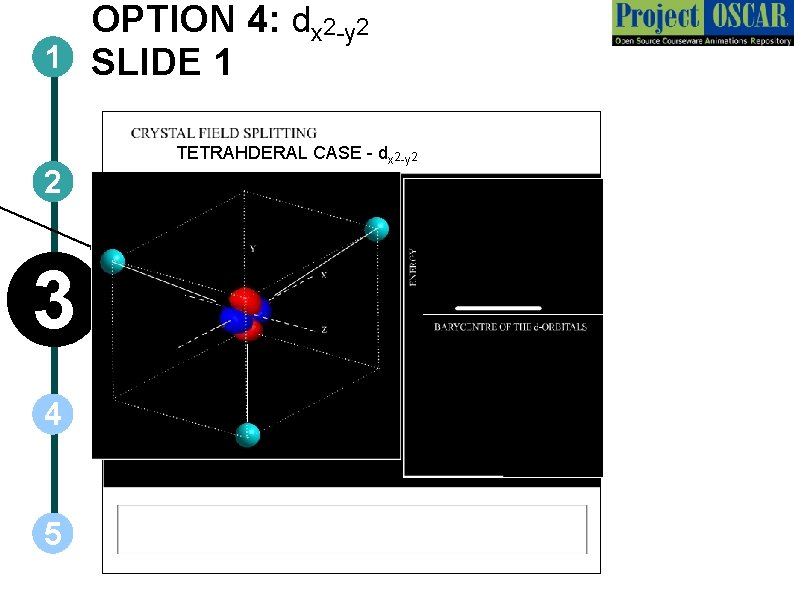
OPTION 4: dx 2 -y 2 1 SLIDE 1 2 3 4 5 TETRAHDERAL CASE - dx 2 -y 2
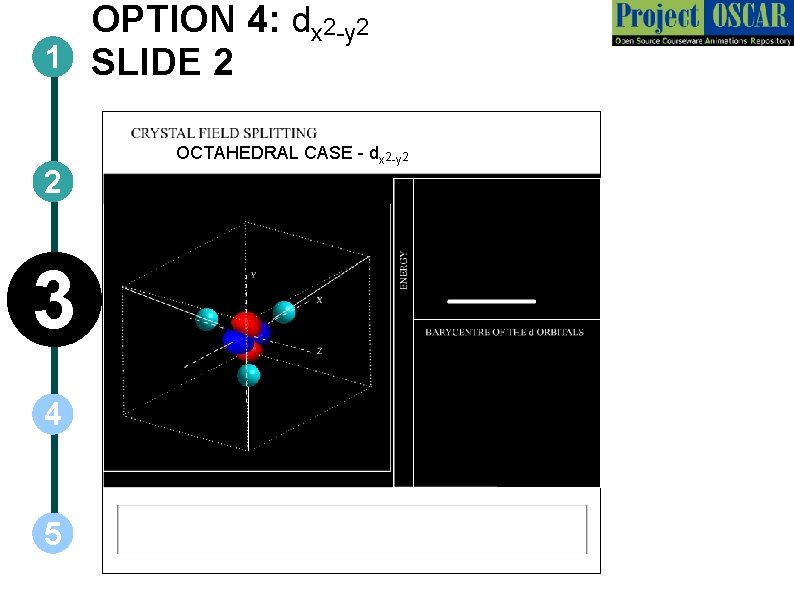
OPTION 4: dx 2 -y 2 1 SLIDE 2 2 3 4 5 OCTAHEDRAL CASE - dx 2 -y 2
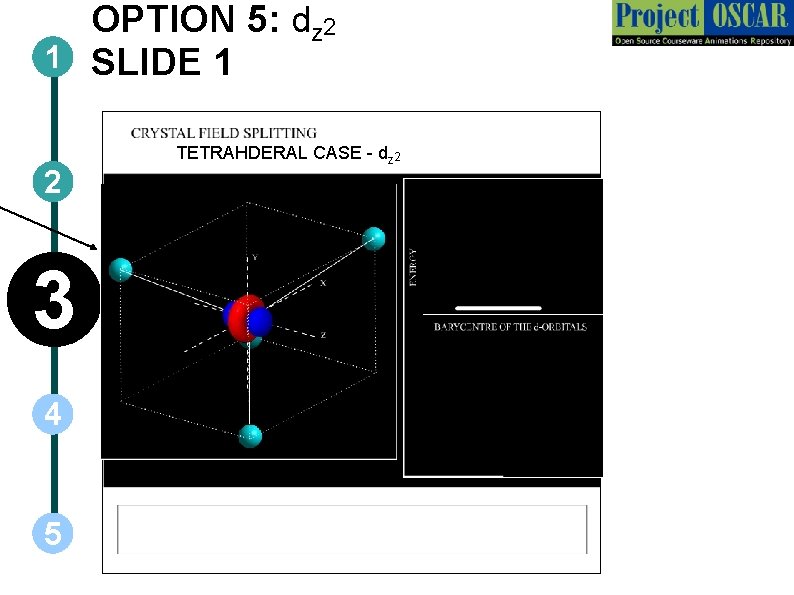
OPTION 5: dz 2 1 SLIDE 1 2 3 4 5 TETRAHDERAL CASE - dz 2
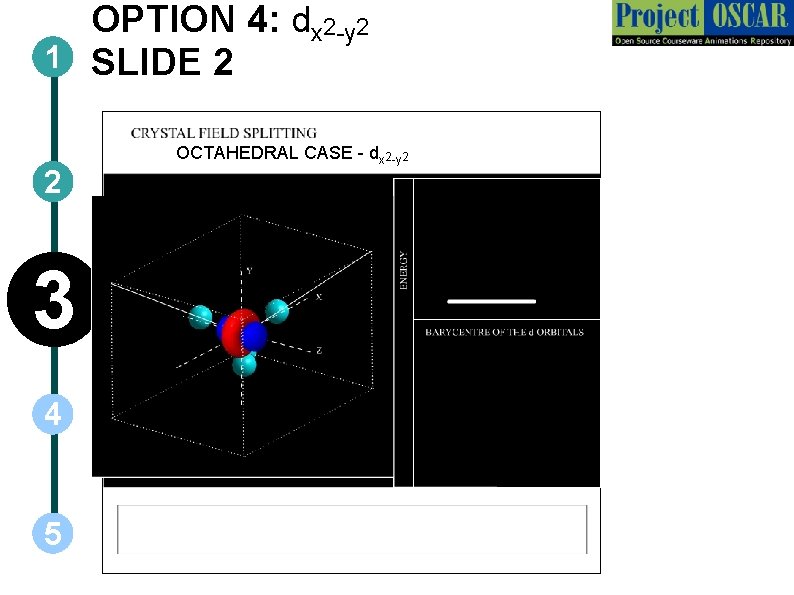
OPTION 4: dx 2 -y 2 1 SLIDE 2 2 3 4 5 OCTAHEDRAL CASE - dx 2 -y 2

Links for further reading Reference websites: http: //www. chemtube 3 d. com/orbitals-d. htm Books: Research papers:
- Slides: 19