Tetanus Diphtheria and Pertussis Tdap Vaccines for Adults

























- Slides: 40

Tetanus, Diphtheria and Pertussis (Tdap) Vaccines for Adults National Immunization Conference March 7, 2007 Susan M. Lett, M. D. , M. P. H Medical Director, Immunization Program Division of Epidemiology and Immunization Massachusetts Department of Public Health

Disclosures n n n The speaker has no financial interest or conflict with the manufacturer of any product named in this presentation The speaker will discuss the use of acellular pertussis vaccines in a manner not approved by the U. S. Food and Drug Administration The speaker may discuss vaccines not currently licensed by the FDA MDPH 3 -07

Acknowledgements n n Katrina Kretsinger, MD, MA, (CDC) William Atkinson, MD, MPH (CDC) Members of the ACIP Tdap Adult Working Group California Immunization Branch MDPH 3 -07

Outline n Pertussis in adults n Tdap vaccines n Recommendation for adults n Recommendations for adolescents n Challenges to introduction MDPH 3 -07

Reported Pertussis Cases – United States, 1922 -2005* > 18 yrs 11 -18 yrs DTP < 11 yrs DTa. P Tdap *1950 -2005, National Notifiable Diseases Surveillance System and 1922 -1949, passive reports MDPH 3 -07 to the Public Health Service, courtesy of Kristin Brown (Presented by K. Kretsinger CDC Satellite Course 1 -18 -07)

Pertussis Among Adolescents and Adults (1) n Broad spectrum of presentation in this age group • Disease can milder than in infants and children • ? May be asymptomatic • But can be quite severe and present as classic pertussis n n Persons with mild disease may transmit the infection Adults often source of infection for children and infants MDPH 3 -07

Pertussis Among Adolescents and Adults (2) n Upper respiratory illness x 1 -2 weeks, followed by cough illness • Median duration of cough illness >2 months n n High risk groups for pertussis not well defined Antimicrobials do not modify the course of illness after cough established • Will decrease infectivity of patients if given early Can result in repeated medical visits and time lost from school and work Impact on public health system MDPH 3 -07

Clinical Manifestations: Adolescents and Adults, MA Paroxysms Vomiting Shortness of breath Urinary incontinence Rib fracture Weight loss LOC Lee et al. , CID 2004 Adolescents (N=314) 74% 56% 72% 3% 1% 33% 1% Adults (N=203) 84% 54% 86% 28% 4% 33% 6% MDPH 3 -07

Pertussis Morbidity, MA n Adolescents: • 38% still coughing at 106 days • 83% missed school for 5. 5 [0. 4 -32] days • 43% parents missed work for 2. 4 [0. 1 -25] days n Adults: • 61% still coughing at 94 days • 61% missed work for 9. 8 [0. 1 -180] days Lee et al. , CID 2004 MDPH 3 -07

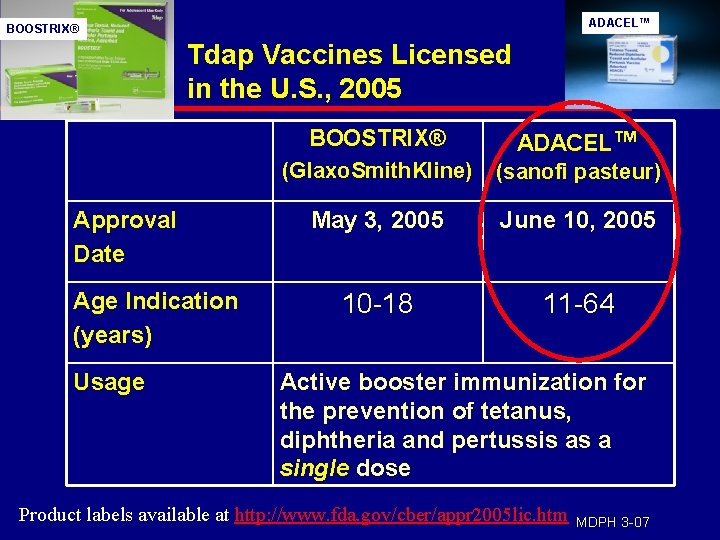
ADACEL™ BOOSTRIX® Tdap Vaccines Licensed in the U. S. , 2005 Approval Date Age Indication (years) Usage BOOSTRIX® (Glaxo. Smith. Kline) ADACEL™ (sanofi pasteur) May 3, 2005 June 10, 2005 10 -18 11 -64 Active booster immunization for the prevention of tetanus, diphtheria and pertussis as a single dose Product labels available at http: //www. fda. gov/cber/appr 2005 lic. htm MDPH 3 -07

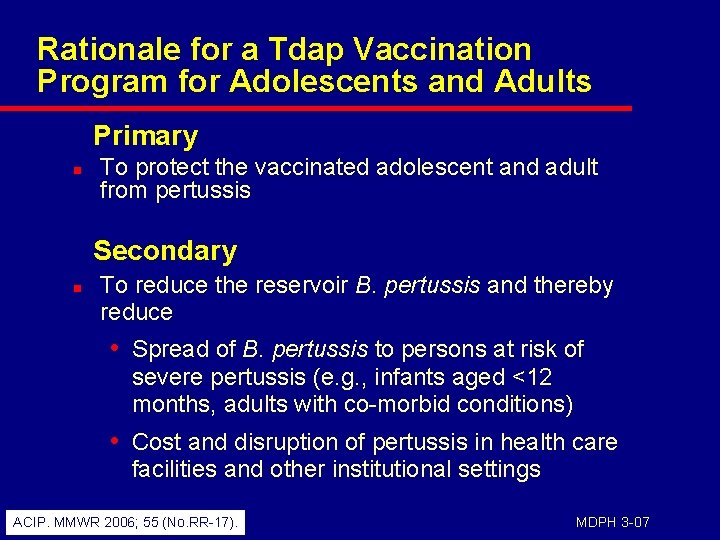
Rationale for a Tdap Vaccination Program for Adolescents and Adults Primary n To protect the vaccinated adolescent and adult from pertussis Secondary n To reduce the reservoir B. pertussis and thereby reduce • Spread of B. pertussis to persons at risk of severe pertussis (e. g. , infants aged <12 months, adults with co-morbid conditions) • Cost and disruption of pertussis in health care facilities and other institutional settings ACIP. MMWR 2006; 55 (No. RR-17). MDPH 3 -07

http: //www. cdc. gov/mmwr/PDF/rr/rr 5517. pdf MDPH 3 -07

Provisional ACIP recommendations for the use of Tdap vaccine in pregnant women (June 30, 2006) and can be found at: http: //www. cdc. gov/nip/recs/provisional_recs/tdap-preg. pdf When the ACIP recommendations for use of Tdap vaccine in pregnant women are final they will be published in the MMWR and will also be posted on the ACIP website: http: //www. cdc. gov/nip/publications/acip-list. htm MDPH 3 -07

ACIP Recommendations for Tdap Adults - Routine n n ADACEL™ is the only Tdap licensed for adults Routine recommendations apply to adults who • Are aged 19 -64 years • No product available for those > 65 years – Safety and immunogenicity not studied – Trials underway n n Licensed for use as a single booster dose • No additional doses recommended at this time Can be given simultaneously with other vaccines MMWR 2006; 55(RR-17). MDPH 3 -07

ACIP Recommendations for Tdap Adults - Routine n n n Single dose of Tdap to replace a single dose of Td May be given at an interval <10 years since receipt of last tetanus toxoid-containing vaccine Special emphasis on adults with close contact with infants: • Parents • Grandparents <65 years of age • Childcare providers • Healthcare workers MMWR 2006; 55(RR-17). MDPH 3 -07

No Minimum Interval Between Td and Tdap n n 1 ACIP did not define an absolute minimum interval between Td and Tdap 1, 2 Providers will need to decide based on whether the benefit of pertussis immunity outweighs the risk of a local adverse reaction Safety of interval as short as 2 years supported by 3 Canadian studies (n>6, 000) off-label recommendation. See MMWR 2006; 55(RR-3), MMWR 2006; 55(RR-17) and ACIP provisional recs MDPH 3 -07 Tda. P and pregnant women 8 -01 -06 ttp: //www. cdc. gov/nip/recs/provisional_recs/tdap-preg. pdf 2

Short Interval Between Td and Tdap (2) n Providers can consider short intervals 1, 2 between Td and Tdap: • During increased community activity • During outbreaks • Individuals with comorbid conditions – e. g. , chronic cardiovascular disease, HIV infection, neuromuscular disorders • Household contacts and caregivers of infants < 12 months • Healthcare workers 1 Safety of interval as short as 2 years supported by 3 Canadian studies (n>6, 000) 2 off-label recommendation. See MMWR 2006; 55(RR-3), MMWR 2006; 55(RR-17) and ACIP provisional recs MDPH 3 -07 Tda. P and pregnant women 8 -01 -06 ttp: //www. cdc. gov/nip/recs/provisional_recs/tdap-preg. pdf

Use of Tdap to Protect Infants ‘Cocoon Strategy’ n Adults who have or who anticipate having close contact with an infant aged <12 months should receive a single dose of Tdap • An interval as short as 2 years from last Td suggested – But shorter interval may be used n n n • Ideally give > 2 weeks before contact with the infant. Infants should receive DTa. P on schedule When possible, women should receive Tdap before conception. Pregnant women should receive Tdap in the immediate post-partum period. MMWR 2006; 55(RR-17). MDPH 3 -07

Tdap Vaccine and Healthcare Workers (1) n n n Health-care personnel (HCP) in hospitals* or ambulatory care settings who have direct patient contact should receive a single dose of Tdap as soon as feasible. Priority should be given to HCP in contact with infants <12 months An interval as short as 2 years (or less) from the last Td is recommended for the Tdap dose *Hospitals, as defined by the Joint Commission on Accreditation of Healthcare Organizations, do not include long term care facilities such as nursing homes, skilled nursing facilities, rehabilitation and convalescent facilities. Ambulatory care settings include all outpatient and walk-in facilities. MMWR 2006; 55(RR-17). MDPH 3 -07

Tdap Vaccine and Healthcare Workers (2) n n n Hospitals and ambulatory care facilities should provide Tdap for HCP Facilities should use approaches that maximize vaccination rates • Education about benefits • Convenient access Recommendations supported by Healthcare Infection Control Practices Advisory Committee (HICPAC) MMWR 2006; 55(RR-17). MDPH 3 -07

Tdap and Pregnancy* n n n Td is generally preferred during pregnancy Data on safety and immunogenicity not yet available Transplacental maternal antibodies might: • Provide protection to infants in 1 st few months of life • Could also interfere will infant’s immune response to DTa. P n n A clinician may choose to administer Tdap to a pregnant woman in certain circumstances • exposure or transmission risks Pregnancy is not a contraindication to vaccination with Tdap** *, ** Off-label recommendation. See ACIP provisional recs Tda. P and pregnant women 8 -01 -06 MDPH 3 -07 http: //www. cdc. gov/nip/recs/provisional_recs/tdap-preg. pdf

Considerations for Use of Tdap in Pregnant Women (1)* n n n Tdap can be used in pregnant women at increased risk of exposure due to increased pertussis activity in • Institution where employed • Community where living Tdap can also be used in pregnant women to prevent transmission to infants and other vulnerable groups • Health care workers • Child care workers Tdap can also be used instead of Td for routine use of ‘catch-up’ in pregnant adolescents to prevent transmission to young infants • Because incidence of pertussis is high in adolescents * Off-label recommendation. See ACIP provisional recs Tdap and pregnant women 8 -01 -06 MDPH 3 -07 http: //www. cdc. gov/nip/recs/provisional_recs/tdap-preg. pdf

Considerations for Use of Tdap in Pregnant Women (2)* n n n When Tdap is used instead of Td, the 2 nd or 3 rd trimester is preferred Providers should discuss the lack of data with the patient Because of the lack of data on the use of Tdap in pregnant women, both manufacturers have established registries and providers should report any Tdap administration to a pregnant woman • BOOSTRIX® (Glaxo. Smith Kline): 888 -825 -5249 • ADACEL™ (sanofipasteur) 800 -822 -2463) * ACIP provision recs Tdap and pregnant women 8 -01 -06 http: //www. cdc. gov/nip/recs/provisional_recs/tdap-preg. pdf MDPH 3 -07

http: //www. cdc. gov/mmwr/PDF/rr/rr 55 e 223. pdf MDPH 3 -07

ACIP Recommendations for Tdap Adolescents n Adolescents 11 -12 years of age should receive single dose of Tdap (instead of Td), if they have completed the recommended childhood DTa. P vaccination series • Those 13 -18 years of age who have not yet received a Td should receive a single dose also. n n Adolescents 11 -18 years who have already received Td are encouraged to receive a single dose of Tdap, to provide protection against pertussis, if they have completed the recommended childhood DTa. P vaccination series A 5 year interval is encouraged to reduce the chance of a local reaction MMWR 2006; 55(RR-3). MDPH 3 -07

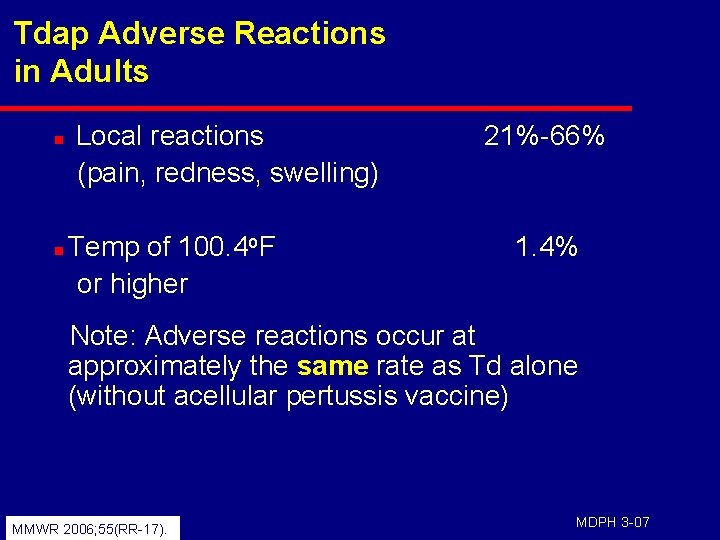
Tdap Adverse Reactions in Adults n n Local reactions (pain, redness, swelling) Temp of 100. 4 o. F or higher 21%-66% 1. 4% Note: Adverse reactions occur at approximately the same rate as Td alone (without acellular pertussis vaccine) MMWR 2006; 55(RR-17). MDPH 3 -07

Tdap Contraindications n Severe allergic reaction to a vaccine component or following a prior dose • ADACEL™ does not contain thimerosal or latex n Encephalopathy (e. g. , coma or prolonged seizures) within 7 days of administration of a pertussis vaccine that is not attributable to another identifiable cause* * Contraindication for pertussis component; Td can be used MMWR 2006; 55(RR-17). MDPH 3 -07

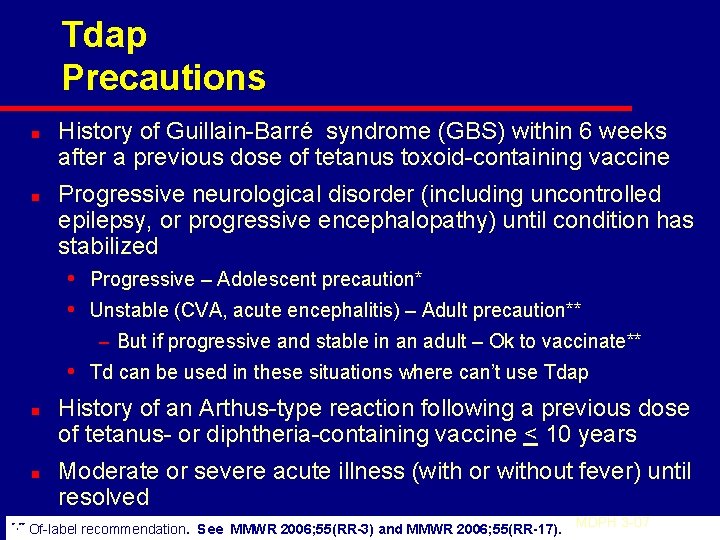
Tdap Precautions n n History of Guillain-Barré syndrome (GBS) within 6 weeks after a previous dose of tetanus toxoid-containing vaccine Progressive neurological disorder (including uncontrolled epilepsy, or progressive encephalopathy) until condition has stabilized • Progressive – Adolescent precaution* • Unstable (CVA, acute encephalitis) – Adult precaution** – But if progressive and stable in an adult – Ok to vaccinate** • Td can be used in these situations where can’t use Tdap n n *, ** History of an Arthus-type reaction following a previous dose of tetanus- or diphtheria-containing vaccine < 10 years Moderate or severe acute illness (with or without fever) until resolved Of-label recommendation. See MMWR 2006; 55(RR-3) and MMWR 2006; 55(RR-17). MDPH 3 -07

Selected Special Situations Situation Recommendations for adults and adolescents who have not received Tdap Pertussis outbreak Tdap can be used at shorter intervals since most recent Td History of Pertussis Use Tdap Wound Tdap preferred to Td if tetanus prophylaxis management indicated; use standard algorithm Incomplete schedule MMWR 2006; 55(RR-17). Use Tdap as one of the doses for catch-up (1 st dose preferred); use Td for other doses MDPH 3 -07 (Presented by K. Kretsinger CDC Satellite Course 1 -18 -07)

Resource to Help Reduce DTa. P/Tdap Administration Errors ‘Check Your Vials’ poster showing images of DTa. P/Tdap/Td vials and packaging Developed by the California Immunization Branch available at: www. dhs. ca. gov/ps/dcdc/izgroup/pdf/IMM-508. pdf MDPH 3 -07

Challenges to Tdap Implementation (1) n n Funding • Public sector • Private sector Adult providers • Some new partners – not familiar with ordering, storing and administering vaccines MDPH 3 -07

Challenges to Tdap Implementation (2) n n n Hosptials • Healthcare workers • Postpartum women Use of Tdap post exposure • Operational and funding challenges Management of Tdap recipients who are exposed to pertussis • Healthcare setting – some guidelines in ACIP statement – Prophylax according to usual recommendations – Option to do monitoring in nonhigh-risk settings for symptoms ( e. g. , varicella and smallpox vaccination) • Other settings – Should receive antibiotic prophylaxis MDPH 3 -07

QUESTIONS? MDPH 3 -07

EXTRA SLIDES MDPH 3 -07

Pertussis Transmission to Infants n Infants <12 months of age greatest risk for death and complications from pertussis • From 2000 -2004 – Accounted for 92 out of 100 U. S. pertussis deaths – Risk of death highest among youngest infants – Over 60% infants with pertussis hospitalized n Adults transmit to infants* • Among 264 known source-cases – 55% identified as mother, father or grandparent – 51% were adults >19 years of age Bisgard KM, et. al Pediatr Infect Dis J 2004. MDPH 3 -07 (Presented by K. Kretsinger CDC Satellite Course 1 -18 -07)

ADACEL® Safety Rates of Selected Solicited Adverse Events In Adults Aged 18─64 Within 15 Days After a Single Dose of Tdap or Td MDPH 3 -07 Source: Product label available at http: //www. vaccineplace. com/products/

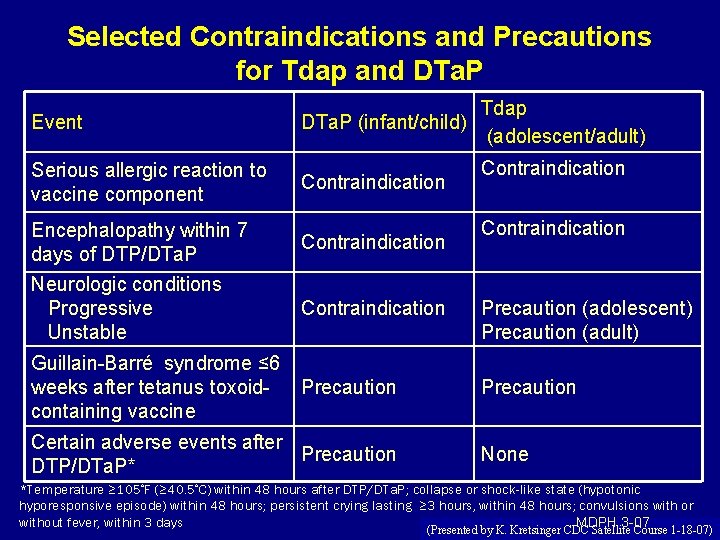
Selected Contraindications and Precautions for Tdap and DTa. P Event Serious allergic reaction to vaccine component Encephalopathy within 7 days of DTP/DTa. P Neurologic conditions Progressive Unstable Guillain-Barré syndrome ≤ 6 weeks after tetanus toxoidcontaining vaccine Tdap DTa. P (infant/child) (adolescent/adult) Contraindication Contraindication Precaution (adolescent) Precaution (adult) Precaution Certain adverse events after Precaution DTP/DTa. P* None *Temperature ≥ 105◦F (≥ 40. 5◦C) within 48 hours after DTP/DTa. P; collapse or shock-like state (hypotonic hyporesponsive episode) within 48 hours; persistent crying lasting ≥ 3 hours, within 48 hours; convulsions with or MDPH 3 -07 without fever, within 3 days (Presented by K. Kretsinger CDC Satellite Course 1 -18 -07)

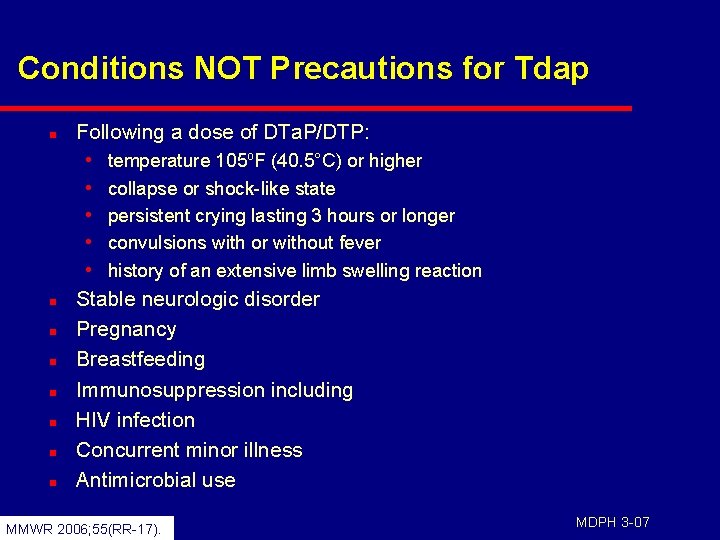
Conditions NOT Precautions for Tdap n Following a dose of DTa. P/DTP: • • • n n n n temperature 105 o. F (40. 5°C) or higher collapse or shock-like state persistent crying lasting 3 hours or longer convulsions with or without fever history of an extensive limb swelling reaction Stable neurologic disorder Pregnancy Breastfeeding Immunosuppression including HIV infection Concurrent minor illness Antimicrobial use MMWR 2006; 55(RR-17). MDPH 3 -07

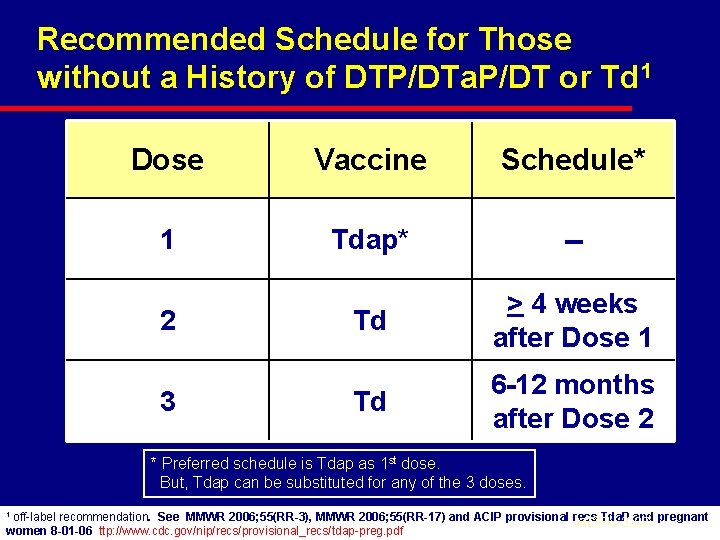
Recommended Schedule for Those without a History of DTP/DTa. P/DT or Td 1 Dose Vaccine Schedule* 1 Tdap* -- Td > 4 weeks after Dose 1 Td 6 -12 months after Dose 2 2 3 * Preferred schedule is Tdap as 1 st dose. But, Tdap can be substituted for any of the 3 doses. 1 off-label recommendation. See MMWR 2006; 55(RR-3), MMWR 2006; 55(RR-17) and ACIP provisional recs Tda. P and pregnant MDPH 3 -07 women 8 -01 -06 ttp: //www. cdc. gov/nip/recs/provisional_recs/tdap-preg. pdf

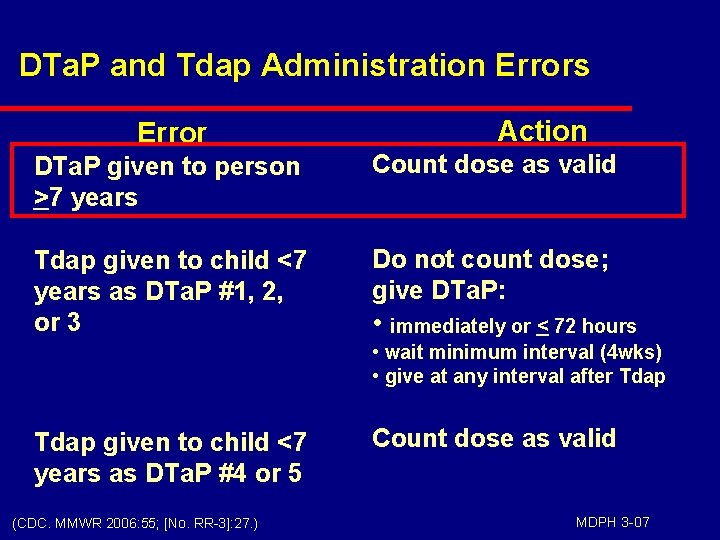
DTa. P and Tdap Administration Errors Error Action DTa. P given to person >7 years Count dose as valid Tdap given to child <7 years as DTa. P #1, 2, or 3 Do not count dose; give DTa. P: • immediately or < 72 hours • wait minimum interval (4 wks) • give at any interval after Tdap given to child <7 years as DTa. P #4 or 5 (CDC. MMWR 2006: 55; [No. RR-3]: 27. ) Count dose as valid MDPH 3 -07
 Tdap vaccine kroger
Tdap vaccine kroger Pertussis etymology
Pertussis etymology Preventive measures of diphtheria
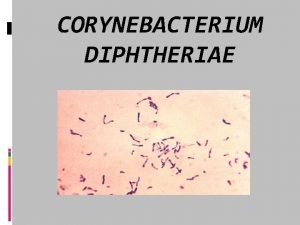
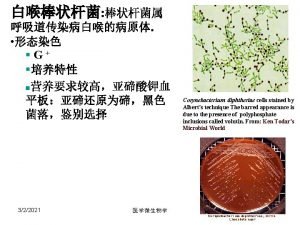
Preventive measures of diphtheria Corynebacterium diphtheriae gram stain morphology
Corynebacterium diphtheriae gram stain morphology Diphtheria
Diphtheria Diphtheria cdc
Diphtheria cdc Diphtheria
Diphtheria Virulent
Virulent Edible vaccines pros and cons
Edible vaccines pros and cons Meaning of variolation
Meaning of variolation Global alliance for vaccines and immunization
Global alliance for vaccines and immunization Edible vaccines pros and cons
Edible vaccines pros and cons Is glyphosate in vaccines
Is glyphosate in vaccines Edible vaccines in pharmacognosy
Edible vaccines in pharmacognosy Stephanie seneff mit
Stephanie seneff mit Immune checkpoint inhibitors mechanism of action
Immune checkpoint inhibitors mechanism of action Tuberculose transmission
Tuberculose transmission Could vaccines breed viciousness
Could vaccines breed viciousness Hep b vaccines
Hep b vaccines Virulent
Virulent Www.cdc.gov/vaccines/schedules/index.html
Www.cdc.gov/vaccines/schedules/index.html History of vaccines pdf
History of vaccines pdf Brighton collaboration criteria anaphylaxis
Brighton collaboration criteria anaphylaxis Spacing out vaccines
Spacing out vaccines Neuretic
Neuretic Dr fajar maskuri
Dr fajar maskuri Derajat trismus
Derajat trismus Incomplete tetanus muscle contraction
Incomplete tetanus muscle contraction What is staircase phenomenon
What is staircase phenomenon Geburtenkontrolle durch impfung
Geburtenkontrolle durch impfung Hondenbeet tetanus wanneer
Hondenbeet tetanus wanneer Direct phosphorylation
Direct phosphorylation Tetanus vs summation
Tetanus vs summation Charles bell tetanus
Charles bell tetanus Risus sardonicus
Risus sardonicus Prophalaxysis
Prophalaxysis Tetanus
Tetanus Osha log posting dates
Osha log posting dates Tetanus
Tetanus Tetanus
Tetanus Sarkolema
Sarkolema