Tests for positive ions Do Now Activity Use







- Slides: 11

Tests for positive ions Do Now Activity: Use the periodic table to find the symbols for the following metals: 1. Lithium Li+ 6. Aluminium Al 3+ 2. Sodium Na+ 7. Calcium Ca 2+ 3. Potassium K+ 8. Magnesium Mg 2+ 4. Calcium Ca 2+ 9. Iron (II) 5. Copper (II) Cu 2+ 10. Iron (III) Fe 2+ Fe 3+ EXTENSION! Can you work out the charge of the ion that each metal forms? HINT – Look at the group number

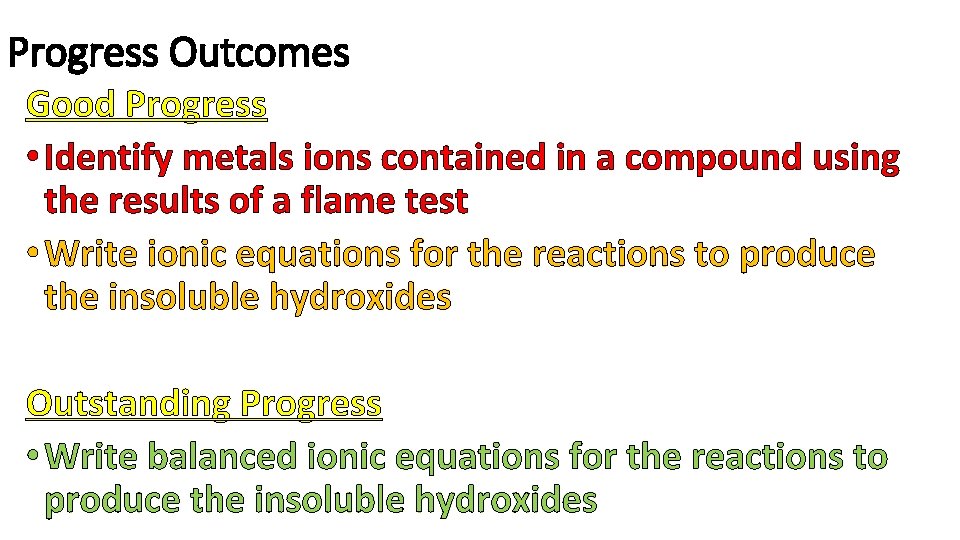
Progress Outcomes Good Progress • Identify metals ions contained in a compound using the results of a flame test • Write ionic equations for the reactions to produce the insoluble hydroxides Outstanding Progress • Write balanced ionic equations for the reactions to produce the insoluble hydroxides

3 of 41 © Boardworks Ltd 2009

Method 1. 2. 3. 4. 5. 6. Flame tests Pour around 1 cm depth of each of the labelled chloride solutions into five test tubes in the rack. Dip the nichrome wire into the first solution. Then hold the tip of the wire in a blue Bunsen burner flame. Record your observation in the first row of Table 1. Clean the wire carefully. Repeat steps 2‒ 4 for each of the other four solutions. Empty and clean the test tubes.

Method 1. 2. 3. 4. 5. 6. Hydroxide tests Pour around 1 cm depth of each of the labelled chloride solutions into five test tubes in the rack. Add a little sodium hydroxide and record any precipitation (solid within the solution) Record your observation in the second row of Table 1. Repeat steps 2‒ 3 for each of the other four solutions. Empty and clean the test tubes. You will have to add the results of ‘Aluminium’ ‘Magnesium’ ‘Iron (II)’ and ‘Iron (III)’ on the back of your sheet.

6 of 41 © Boardworks Ltd 2009

Plenary 7 of 41 © Boardworks Ltd 2009

Practical Carry out the flame tests to work out how the metal ion changes the flame colour Positive Flame ion colour Lithium Crimson Sodium Yellow Potassium Lilac Calcium Orange-red Green Copper

Practical Use sodium hydroxide to test for the following cations: Positive ion Aluminium Calcium Magnesium Copper (II) Iron (III) Colour of precipitate White at first White Blue Green Brown Redissolves in excess Na. OH to form a colourless solution Positive ion Lithium Sodium Potassium Colour of precipitate White

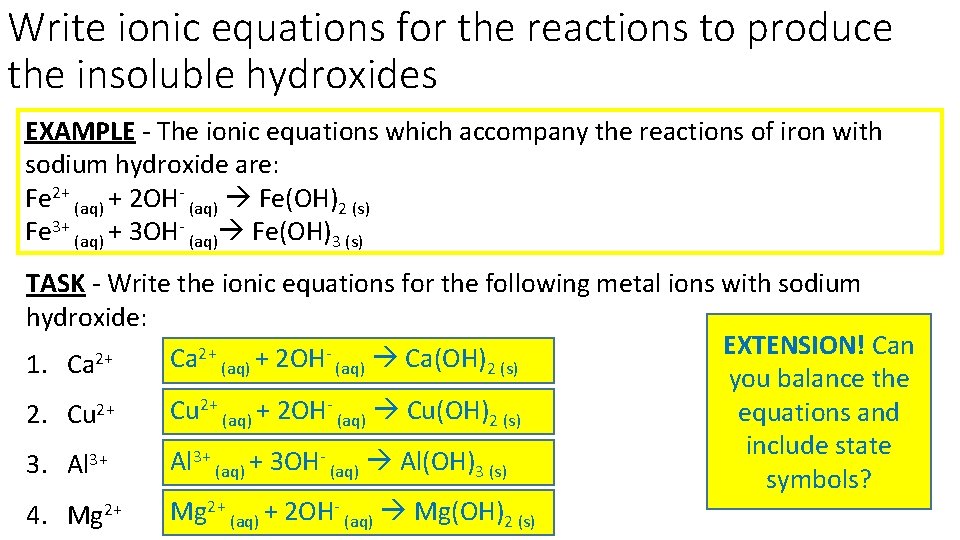
Write ionic equations for the reactions to produce the insoluble hydroxides EXAMPLE - The ionic equations which accompany the reactions of iron with sodium hydroxide are: Fe 2+ (aq) + 2 OH- (aq) Fe(OH)2 (s) Fe 3+ (aq) + 3 OH- (aq) Fe(OH)3 (s) TASK - Write the ionic equations for the following metal ions with sodium hydroxide: EXTENSION! Can 2+ 2+ Ca + 2 OH Ca(OH) 1. Ca (aq) 2 (s) you balance the Cu 2+ (aq) + 2 OH- (aq) Cu(OH)2 (s) equations and 2. Cu 2+ include state 3+ 3+ Al (aq) + 3 OH (aq) Al(OH)3 (s) 3. Al symbols? Mg 2+ (aq) + 2 OH- (aq) Mg(OH)2 (s) 4. Mg 2+

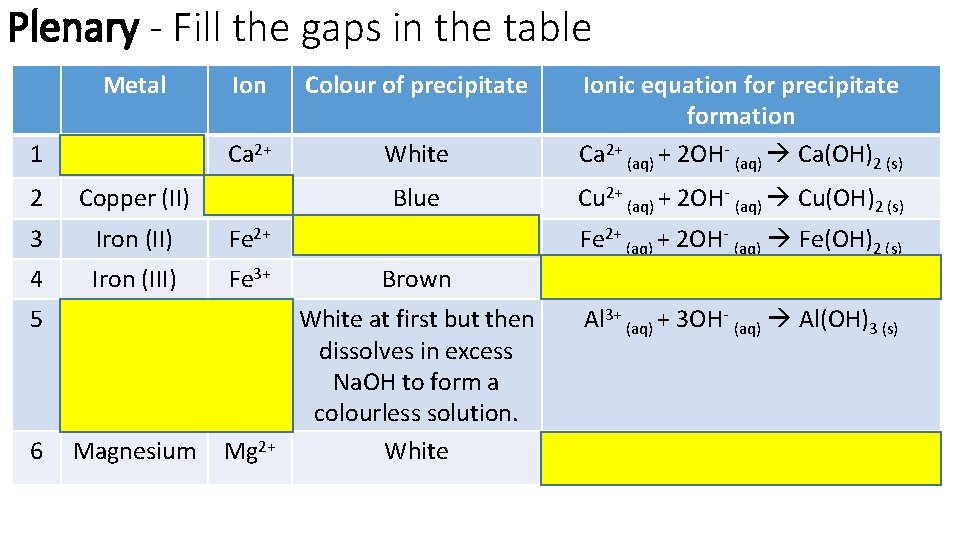
Plenary - Fill the gaps in the table Metal Ion Colour of precipitate 1 Calcium Ca 2+ White Ionic equation for precipitate formation Ca 2+ (aq) + 2 OH- (aq) Ca(OH)2 (s) 2 Copper (II) Cu 2+ Blue Cu 2+ (aq) + 2 OH- (aq) Cu(OH)2 (s) 3 Iron (II) Fe 2+ Green Fe 2+ (aq) + 2 OH- (aq) Fe(OH)2 (s) 4 Iron (III) Fe 3+ Brown Fe 3+ (aq) + 3 OH- (aq) Fe(OH)3 (s) 5 Aluminium Al 3+ (aq) + 3 OH- (aq) Al(OH)3 (s) 6 Magnesium Mg 2+ White at first but then dissolves in excess Na. OH to form a colourless solution. White Mg 2+ (aq) + 2 OH- (aq) Mg(OH)2 (s)