Tests for Positive Ions 2 Qualitative tests Learning










- Slides: 13

Tests for Positive Ions. 2. ) Qualitative tests.

Learning Objectives. By the end of the session: • Everyone will have experience of carrying out qualitative tests for positive ions. • Most students will be able to identify Aluminium, Calcium, Copper (II), Iron (III) Ammonium and Magnesium ions. • A few students will be able to identify an unknown ion using qualitative tests.

Starter. • We have seen that flame tests allow us to identify some metal ions. • If I try to carry out a flame test on a compound containing Aluminium, Magnesium or Lead, what do you notice? What did we notice about the flame colours of Calcium and Lithium? • Some elements have flame colours which are very similar. This makes them difficult to identify. • Today, we will look at another technique to help us differentiate between compounds which give similar flame tests.

Safety. • Sodium hydroxide is corrosive. • Treat all known and unknown ions as if they were toxic. • Ammonia gas is harmful and should not be inhaled. • Care needed when heating Sodium hydroxide solution.

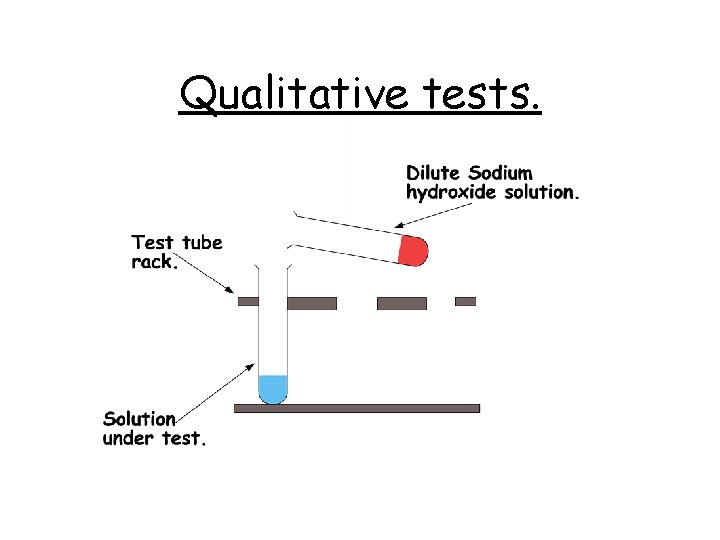
Qualitative tests.

Qualitative tests. Using the apparatus on the previous slide, this is the procedure for qualitative tests. 1. Place approximately 1 cm 3 of the substance under test, in a test tube. 2. Add 1 cm 3 of dilute Sodium Hydroxide solution. 3. Carefully observe. Note your observations. 4. Repeat steps 2 & 3 twice more.

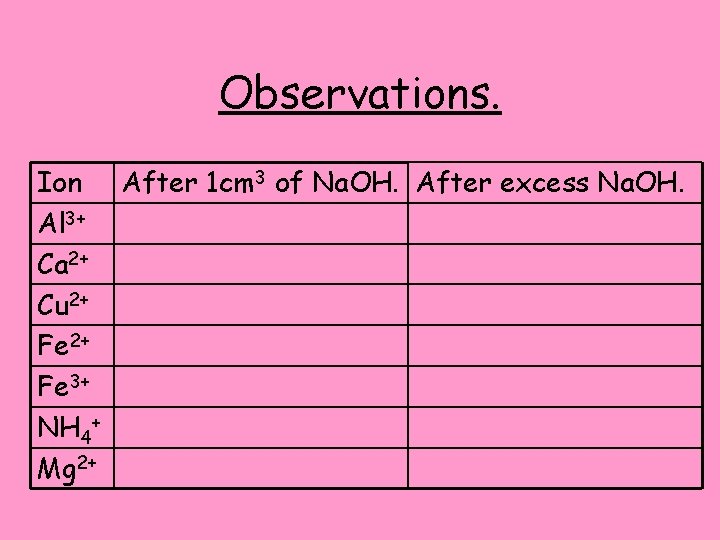
Observations. Ion After 1 cm 3 of Na. OH. After excess Na. OH. Al 3+ Ca 2+ Cu 2+ Fe 3+ NH 4+ Mg 2+

Observations. Ion After 1 cm 3 of Na. OH. After excess Na. OH. Wispy, white precipitate. Precipitate dissolves to give colourless 3+ Al solution. No change. Ca 2+ White precipitate formed. No change. Cu 2+ Blue gelatinous precipitate formed, No change. Fe 2+ Mud-green may turn brown at surface. Rust-brown gelatinous precipitate formed. No change. 3+ Fe No precipitate, faint but pungent smell No change. + NH 4 may be present. No change. Mg 2+ White precipitate formed.

Observations. • • You should now have a complete set of observations for the test-tube reactions. Repeat the test for Ammonium ions, but this time, gently heat the test-tube over a Bunsen burner. Hold a piece of damp red litmus paper to the mouth of the testtube. What do you see? What does this mean? Why moisten the litmus paper?

Unknown substance. • Test the unknown substance with Sodium Hydroxide solution. Note your observations and compare with the results of your experiments. Can you identify the unknown? • X=

Unknown substance. • Test the unknown substance with Sodium Hydroxide solution. Note your observations and compare with the results of your experiments. Can you identify the unknown? • X=

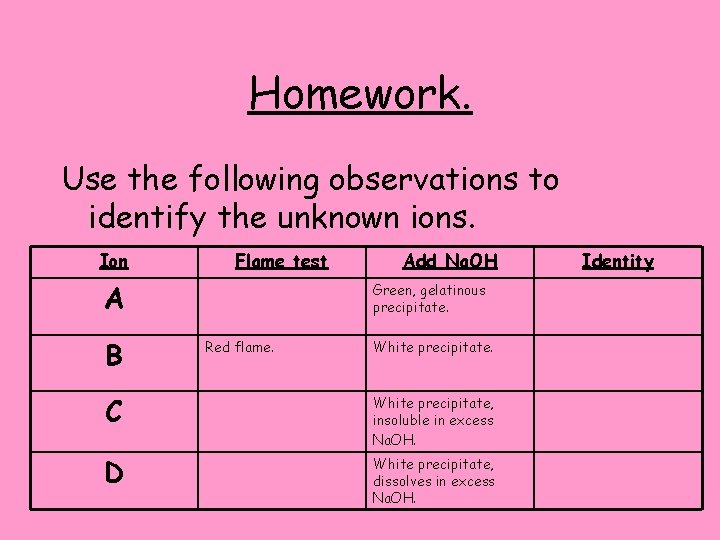
Homework. Use the following observations to identify the unknown ions. Ion Flame test A B Add Na. OH Green, gelatinous precipitate. Red flame. White precipitate. C White precipitate, insoluble in excess Na. OH. D White precipitate, dissolves in excess Na. OH. Identity

Learning Objectives. By the end of the session: • Everyone will have experience of carrying out qualitative tests for positive ions. • Most students will be able to identify Aluminium, Calcium, Copper (II), Iron (III) Ammonium and Magnesium ions. • A few students will be able to identify an unknown ion using qualitative tests.