Tests for Anions Need to know test for







- Slides: 14

Tests for Anions Need to know test for • Chloride • Sulfate/sulfite • carbonate/hydrogen carbonate • nitrate • phosphate • (NB know confirmatory test too!) AG

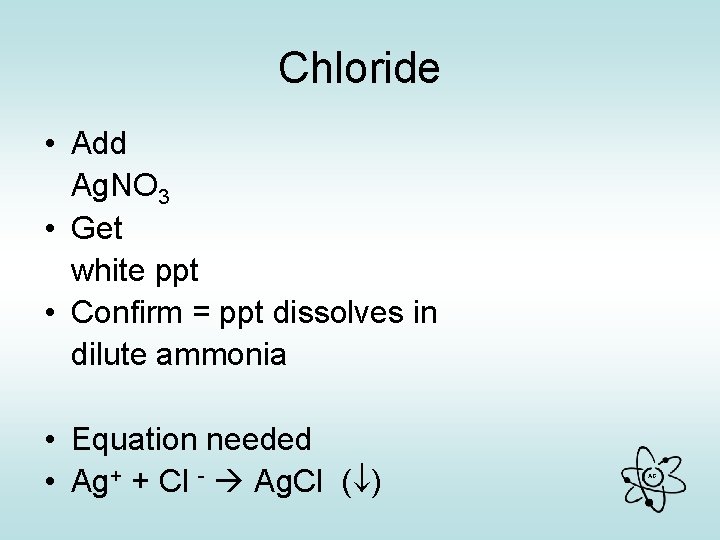
Chloride • Add Ag. NO 3 • Get white ppt • Confirm = ppt dissolves in dilute ammonia • Equation needed • Ag+ + Cl - Ag. Cl ( ) AG

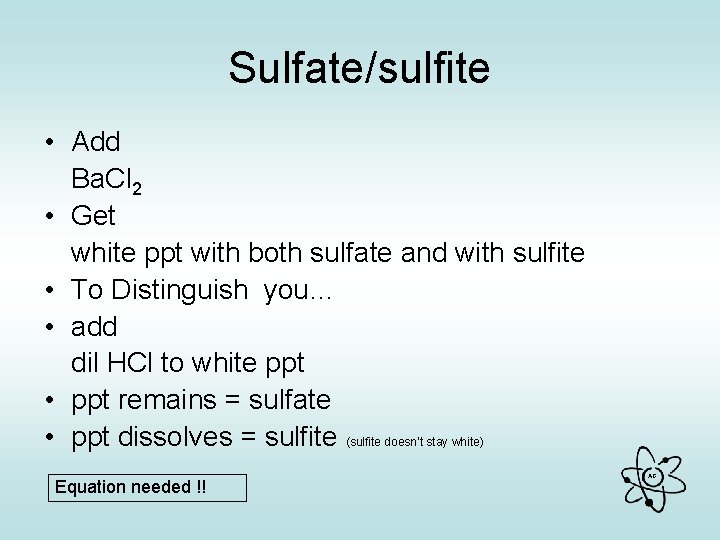
Sulfate/sulfite • Add Ba. Cl 2 • Get white ppt with both sulfate and with sulfite • To Distinguish you… • add dil HCl to white ppt • ppt remains = sulfate • ppt dissolves = sulfite (sulfite doesn’t stay white) Equation needed !! AG

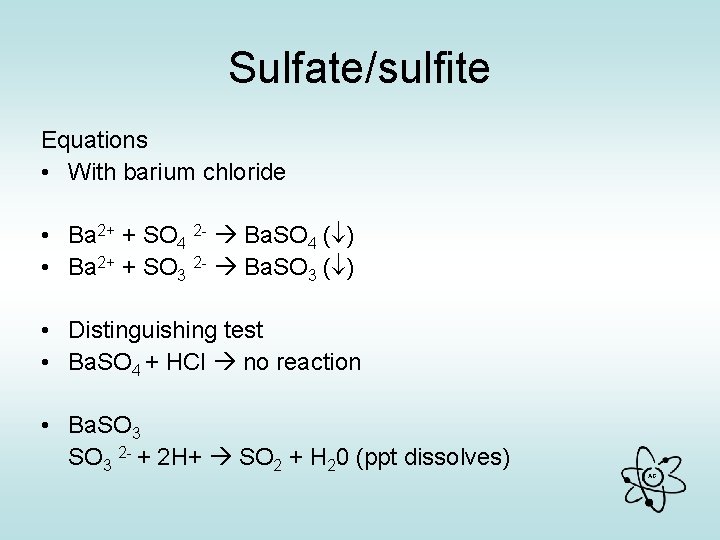
Sulfate/sulfite Equations • With barium chloride • Ba 2+ + SO 4 2 - Ba. SO 4 ( ) • Ba 2+ + SO 3 2 - Ba. SO 3 ( ) • Distinguishing test • Ba. SO 4 + HCl no reaction • Ba. SO 3 2 - + 2 H+ SO 2 + H 20 (ppt dissolves) AG

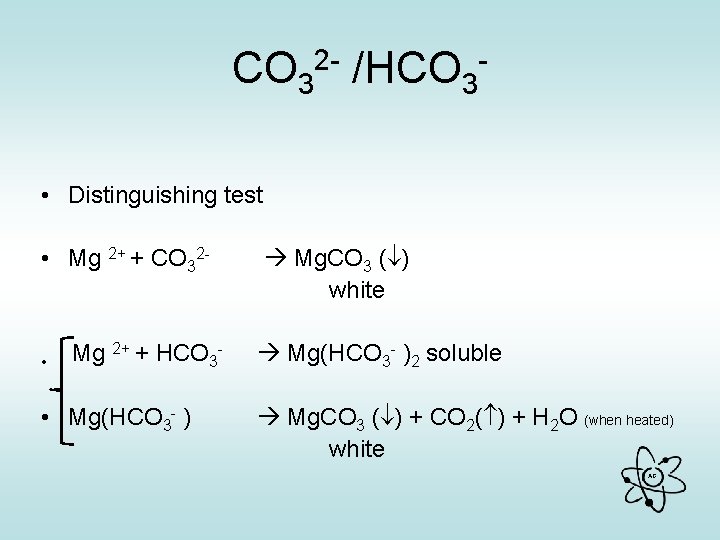
CO 32 - /HCO 3 • Add dil. HCl (or any acid) • Get bubbles of CO 2 (limewater milky) with each • To Distinguish you…. • add Mg. SO 4 to fresh solution • Get white ppt. immediately = carbonate white ppt on heating = hydrogen carbonate Equation needed !! AG

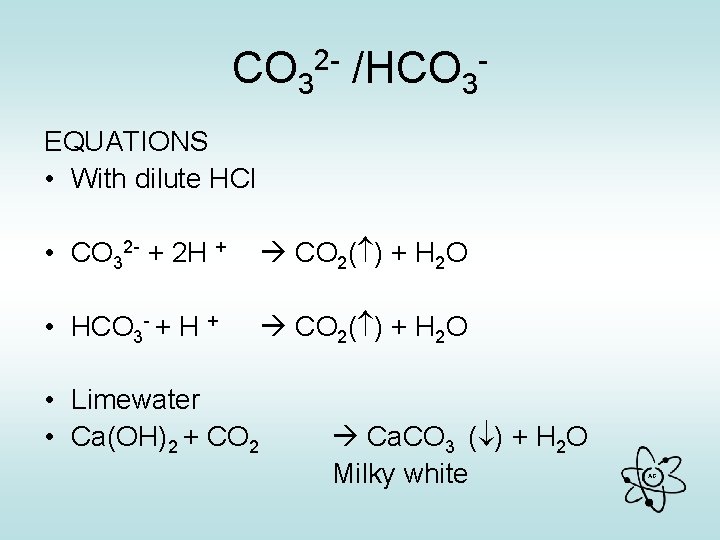
CO 32 - /HCO 3 EQUATIONS • With dilute HCl • CO 32 - + 2 H + CO 2( ) + H 2 O • HCO 3 - + H + CO 2( ) + H 2 O • Limewater • Ca(OH)2 + CO 2 Ca. CO 3 ( ) + H 2 O Milky white AG

CO 32 - /HCO 3 • Distinguishing test • Mg 2+ + CO 32 - • Mg 2+ + HCO 3 - • Mg(HCO 3 - ) Mg. CO 3 ( ) white Mg(HCO 3 - )2 soluble Mg. CO 3 ( ) + CO 2( ) + H 2 O (when heated) white AG

Nitrate • Brown Ring Test • Add fresh Fe. SO 4 At slant add conc. H 2 SO 4 drop wise • Get brown ring at junction of 2 layers No equation needed AG

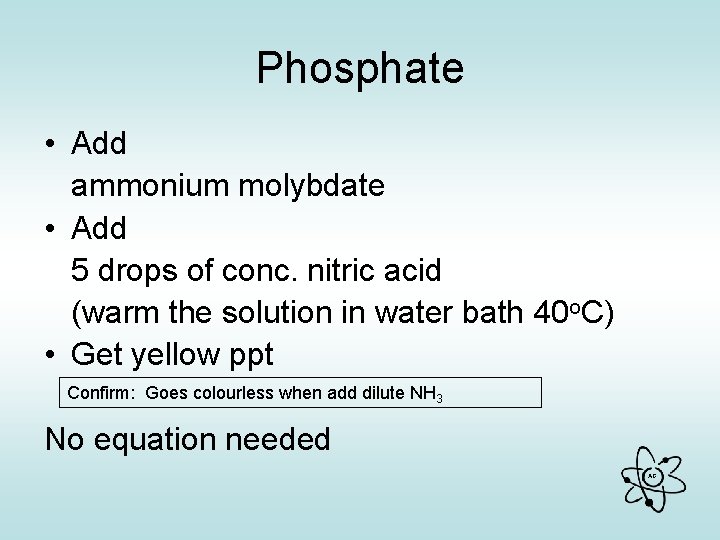
Phosphate • Add ammonium molybdate • Add 5 drops of conc. nitric acid (warm the solution in water bath 40 o. C) • Get yellow ppt Confirm: Goes colourless when add dilute NH 3 No equation needed AG

Why do we need to know? • Nitrates and phosphates are present in fertilisers and detergents • Excess nitrates & phosphates runoff into rivers and lakes • This fertilises the algae – causing algal bloom – lake goes green • Algae die • Dead algae are decomposed by aerobic bacteria • This uses up oxygen • Fish and other animals die • Eutrophication = excess nutrients in water AG

Questions • You add ammonium molybdate when testing for ? • Phosphates • You add silver nitrate when testing for ? • Chlorides • You use Magnesium sulfate for? • Distinguishing between carbonates and hydrogencarbonates • (white ppt formed immediately means carbonate) AG

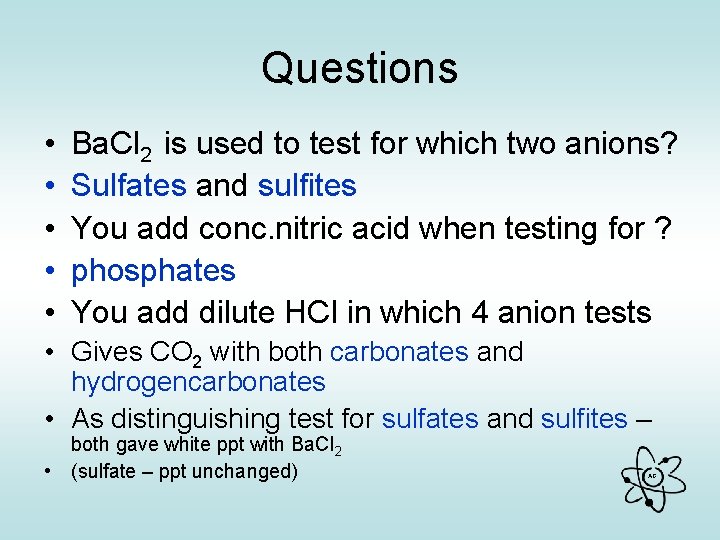
Questions • • • Ba. Cl 2 is used to test for which two anions? Sulfates and sulfites You add conc. nitric acid when testing for ? phosphates You add dilute HCl in which 4 anion tests • Gives CO 2 with both carbonates and hydrogencarbonates • As distinguishing test for sulfates and sulfites – both gave white ppt with Ba. Cl 2 • (sulfate – ppt unchanged) AG

Questions • Ag. NO 3 is used to test for ? • Chloride • You add conclusion can you draw if a white ppt forms when Ba. Cl 2 is added to a solution ? • Solution is either a sulfate or sulfite • What is the confirmatory test for phosphates? • The yellow ppt(from addition of ammonium molybdate, conc HNO 3 and gentle heating ) dissolves in dilute ammonia solution (NH 4 OH) AG

The End! AG