Testing Positive to Contagious Disease Jean Marie Mc


















































































- Slides: 103

Testing Positive to Contagious Disease Jean Marie Mc. Mahon MD

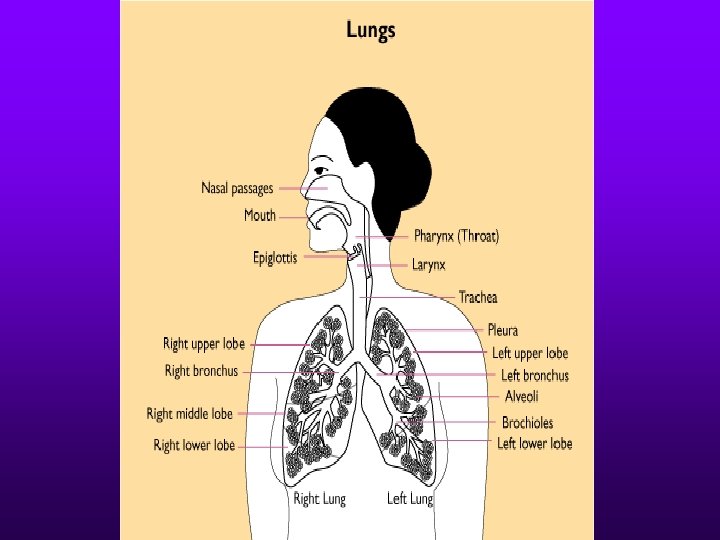
Tuberculosis

Useful Facts about TB Infection • TB is carried in airborne particles called droplet nuclei. • These droplet nuclei are generated when persons who have pulmonary or laryngeal TB disease cough, sneeze, shout or sing. • Normal air currents can keep them airborne for prolonged periods. • Droplet nuclei are approximately 1 -5 um in diameter.


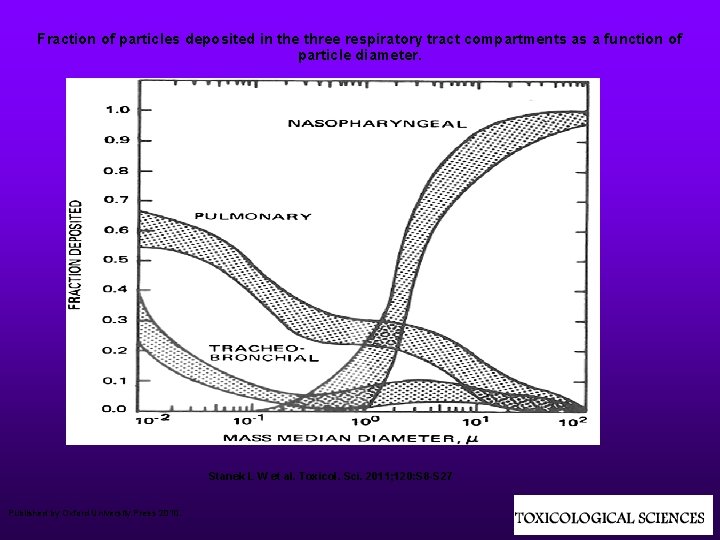
Fraction of particles deposited in the three respiratory tract compartments as a function of particle diameter. Stanek L W et al. Toxicol. Sci. 2011; 120: S 8 -S 27 Published by Oxford University Press 2010.

More Useful Facts about TB Infection • Usually within 2 - 12 weeks after initial infection with M. tuberculosis, the immune response limits additional multiplication of the tubercle bacilli and immunologic test results for M. tuberculosis infection become positive.

The Tuberculin Skin Test (TST) • Although currently available preparations of PPD used in the TST are < 100% sensitive and specific for detection of tuberculosis infection, the TST is currently the most widely used diagnostic test for tuberculosis infection.

Sensitivity of the TST • Sensitivity of a test refers to the percentage of people who have the disease who test positive with the test. • A person with active TB might actually have a negative TST result. • A person with severe problems with their immune system might have a negative TST result.

Specificity of the TST • The specificity of a test refers to the percentage of people who do not have the disease who will have a negative test for the disease. • BCG vaccination • Infection with other types of Mycobacteria

Blood Assay for M. tuberculosis (BAMT) • This test measures the immune response of your blood cells (in a test tube) to two M. tuberculosis proteins that are not present in BCG and that are absent from the majority of non-tuberculosis causing mycobacteria. • More specific than the TST – if you test positive, you are probably infected with M. tuberculosis.

BAMT - Sensitivity • Conditions requiring caution in interpreting negative BAMT’s: • HIV, treatment with immunosuppressive drugs (organ transplantation), TNF-alpha • Diabetes, silicosis, chronic renal failure • Leukemia, lymphoma, cancers of the head, neck or lung

Boosting • Occurs in: • Remote TB infections. • MOTT infection • Previous BCG vaccination

Boosting • In these people, the immune response to mycobacteria may have waned and might not be elicited on the first TST. • A second TST, performed 1 -3 weeks after the 1 st will be positive. • Important: This person did not become infected between tests. He had been infected all along. But if he was not tested using a 2 -step procedure, it will look like he was infected during the interval between the tests.

Baseline Testing for M. tuberculosis infection • The CDC recommends that baseline testing be performed for all newly hired HCW’s, regardless of the risk classification of the setting. • Can be done with TST or BAMT. • If TST chosen, 2 -step testing is recommended for HCW’s whose initial TST results are negative.

Exceptions to the 2 -step Rule • Previous documented negative TST < 12 months before new employment • > 2 previous documented negative TST’s. • Previous documented positive TST

Everyone Else Gets a 2 -step upon Hire • Including: • People who say they have tested positive in the past but cannot document that. • One previous negative TST (documented or not) > 12 months before employment.

Pregnancy • Tens of thousands of pregnant women have received TST without any documented episodes of TST-related fetal harm. • There is no evidence that the TST has adverse effects on the pregnant mother or fetus. • Pregnant HCW’s should be included in serial skin testing as part of an infection control program or a contact investigation. • Guidelines issued by The ACOG emphasize that postponement of the diagnosis of infection with M. tuberculosis during pregnancy is unacceptable.

BCG • • BCG is the most commonly used vaccine in the world. Some people who receive BCG never have a positive result. For others, the positive reaction wanes after 5 years. US guidelines state that a positive TST result in a person who received BCG should be interpreted as indicative of infection. • HCW’s who have previously received a BCG vaccination should receive baseline and serial skin testing in the same manner as those who have not received a BCG vaccination. • This would be a good situation in which to use the BAMT.

Morbidity and Mortality Weekly Report Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Settings, December 30, 2005

Pertinent Excerpts from the New Guidelines. • The new guidelines broaden the scope of settings to which they apply to include “nontraditional facility-based settings. ” • This includes EMS. • The list of “HCW’s Who Should Be Included in a TB Surveillance Program” includes “Patient Transport Staff, including EMS. ”

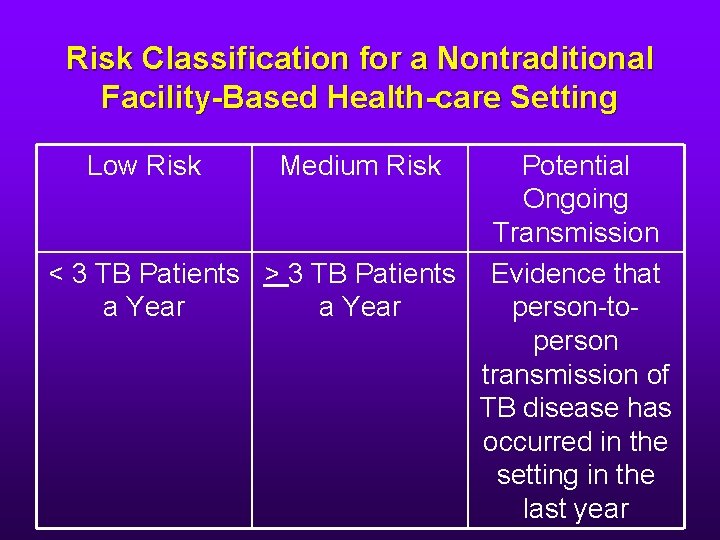
Risk Classification for a Nontraditional Facility-Based Health-care Setting Low Risk Medium Risk Potential Ongoing Transmission < 3 TB Patients > 3 TB Patients Evidence that a Year person-toperson transmission of TB disease has occurred in the setting in the last year

Baseline TST or BAMT • Indicated for new hires to all “nontraditional facility-based settings”, including EMS, regardless of risk classification.

Why the Emphasis on Baseline Testing? • Because the type f medical care that you receive after an exposure is going to be based on a comparison between your baseline testing and your postexposure testing. • There also medico-legal ramifications.

What is an Exposure? • A person with TB is transported who is only later diagnosed with TB. This resulted in failure to apply recommended TB infection controls during transport. • A patient is considered infectious for the 3 month period preceding their diagnosis or beginning with their symptom onset date, whichever is earlier. • Although the overall risk is low, documented transmission of TB has occurred in EMS occupational settings.

How Will I Find Out that I Have Been Exposed? • EMS personnel should be included in the follow up contact investigations of patients with infectious TB disease. • The Ryan White Comprehensive AIDS Resource Emergency Act of 1990 mandates notification of EMS personnel after they have been exposed to a patient with suspected or confirmed TB disease.

You’ve Been Exposed What Now? • You will be assessed for symptoms of TB disease. If you have symptoms of TB, you will be promptly evaluated for active TB disease. This will include a CXR.

You’ve Been Exposed! What Now? • No symptoms, baseline TST was positive: • You do not need to have another TST. Another TST will not provide any additional useful information. • You only need to have a CXR if you are or immunocompromised or otherwise at risk for TB.

You’ve Been Exposed! What Now? • No symptoms, baseline TST negative (0 -9 mm): • You will be given another TST immediately. If this TST is negative, you will be given another TST 8 -10 weeks after exposure ended. • If either of these post-exposure TST’s is positive, you will have a CXR.

What is a “Positive” TST in This Scenario? • If your previous TST result was 0 mm, a post-exposure TST result of > 5 mm will be considered positive and evidence of a new infection. • If your previous TST results was > 0 but < 10 mm, your post-exposure TST will have to increase by 10 mm in order to be considered positive and evidence of a new infection.

You’ve Been Exposed! What Now? • If: • • You have no symptoms, Either of your post-exposure TST’s is positive, and • Your CXR is negative, • You will be diagnosed as having “latent tuberculosis infection” (LTBI)

What Is LTBI? • LTBI is a condition that develops after exposure to a person with infectious TB disease, and subsequent development of infection with M. tuberculosis where the bacteria are still alive but inactive in the body. • Persons with LTBI do not have any symptoms. • They cannot spread TB to other persons.

However • Typically, approximately 5 -10% of persons who become infected with M. tuberculosis (i. e. who have LTBI) will develop TB disease during their lifetimes. • The risk for progression of LTBI to active TB disease is highest during the first several years after infection.

So • If you have been exposed and your TST turns positive (and your CXR is negative), you will be referred to a TB clinic or county health department where you will be evaluated for and encouraged to take treatment for LTBI.

Baseline Testing • You will probably have some baseline blood work performed. • Baseline testing is indicated for: • Persons infected with HIV • Pregnant women • Women in the immediate postpartum period (usually within 3 months of delivery) • Persons with a history of liver disease • Persons who use alcohol regularly • Those who have or are at risk of chronic liver disease.

What Is Treatment for LTBI? • Isoniazid (INH) daily for 9 months (preferred), or • INH twice weekly for 9 months or, • INH daily or twice weekly for 6 months. • If you have acute hepatitis or end stage liver disease, you will not be offered INH. In this case, rifampin daily for 4 months will be offered.

Treatment for LTBI • These regimens might be modified if: • • • You are pregnant You have HIV infection The index case has drug resistant TB

Directly Observed Therapy DOT • Adherence-enhancing strategy in which a HCW or other trained person watches a patient swallow each dose of medication. • DOT is the standard of care for all patients with TB disease and is a preferred option for patients treated for LTBI.

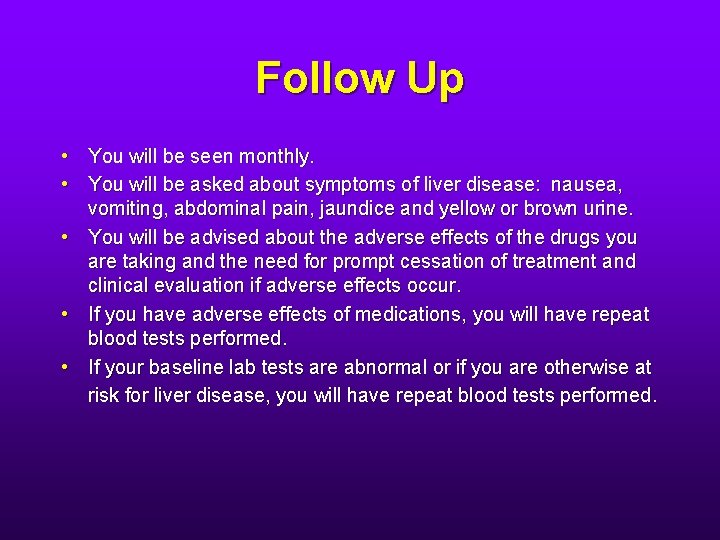
Follow Up • You will be seen monthly. • You will be asked about symptoms of liver disease: nausea, vomiting, abdominal pain, jaundice and yellow or brown urine. • You will be advised about the adverse effects of the drugs you are taking and the need for prompt cessation of treatment and clinical evaluation if adverse effects occur. • If you have adverse effects of medications, you will have repeat blood tests performed. • If your baseline lab tests are abnormal or if you are otherwise at risk for liver disease, you will have repeat blood tests performed.

If You Develop Active TB: • You will probably receive 2 months of four drugs (INH, rifampin, pyrazinamide and ethambutol). • Then, you will receive at least 4 months of INH and rifampin. • DOT is the standard of care and should be used for all doses.

Prevention of Unprotected Exposure • The threat to HCW’s is mainly from patients or others with unsuspected and undiagnosed infectious TB.

Symptoms of Active Infectious TB Disease • Look for symptoms of pulmonary, laryngeal and to a lesser extent, pleural disease. – – – – – Coughing for more than 3 weeks Loss of appetite Weight loss Night sweats Hemoptysis Hoarseness Fever Fatigue Chest pain

Environmental Controls for the Fire Service • The ambulance ventilation system should be operated in the nonrecirculating mode. • The maximum amount of outdoor air should be provided to facilitate dilution. • If the vehicle has a rear exhaust fan, this should be used during transport. • If the vehicle is equipped with a supplemental HEPA filter, use this. • Air should flow from the cab over the patient and out the rear exhaust fan. • If an ambulance is not used, the ventilation system for the vehicle should bring in as much outdoor air as possible and the system should be set to nonrecirculating • If possible, physically isolate the cab from the rest of the vehicle and put the patient in the rear seat

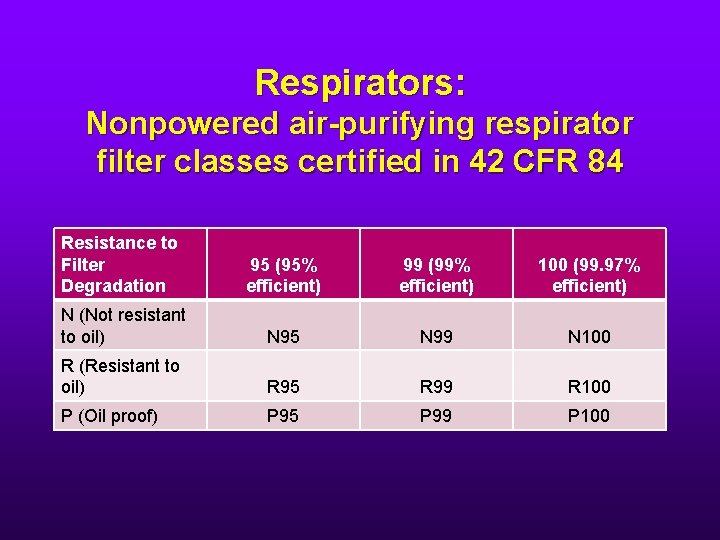
Respirators: Nonpowered air-purifying respirator filter classes certified in 42 CFR 84 Resistance to Filter Degradation 95 (95% efficient) 99 (99% efficient) 100 (99. 97% efficient) N (Not resistant to oil) N 95 N 99 N 100 R (Resistant to oil) R 95 R 99 R 100 P (Oil proof) P 95 P 99 P 100

Respirators • So an N 95 is fine. • It must be fit tested. • You can’t use it with facial hair.

How Long/How Many Times Can I Wear My N 95? • In health care settings in which respirators are used for protection against bioaerosols, the concentration of particles in the air is probably low. Thus, the filter in the respirator is unlikely to become obstructed with airborne material.

How Long/How Many Times Can I Wear My N 95? • Furthermore, no evidence exists to indicate that infectious particles trapped in the filter material of the respirator are reaerosolized easily. • Therefore, the filter material used in respirators in health care settings might remain functional for weeks. • Because electrostatic filter media can degrade, ask the manufacturer about the product’s service life. • But as a general rule, one can use the same N 95 several times a day and discard it after one day’s use.

What’s the Difference Between an N 95 and a Surgical Mask? • An N 95 protects the wearer (the health care worker) from droplet nuclei present in the air space shared with the patient. • A surgical mask does not protect the wearer. It protects others in the shared air space from the wearer’s droplet nuclei. • One cannot be substituted for the other.

Respiratory Controls for the Fire Service • Drivers or other HCWs who are transporting patients with suspected or confirmed infectious TB disease in an enclosed vehicle should wear at least an N 95 disposable respirator. • If the patient has signs of symptoms of infectious TB disease, consider having the patient wear a surgical or procedure mask during transport.

Bloodborne Pathogens Hepatitis B Hepatitis C HIV

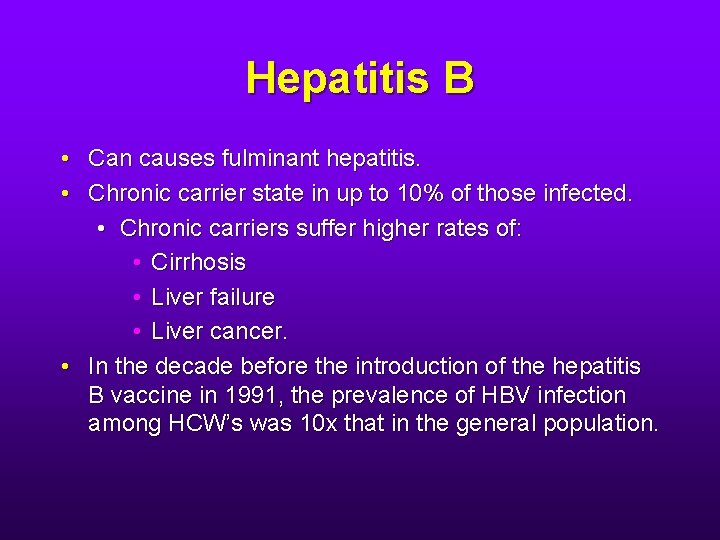
Hepatitis B • Can causes fulminant hepatitis. • Chronic carrier state in up to 10% of those infected. • Chronic carriers suffer higher rates of: • Cirrhosis • Liver failure • Liver cancer. • In the decade before the introduction of the hepatitis B vaccine in 1991, the prevalence of HBV infection among HCW’s was 10 x that in the general population.

Hepatitis C • 70 -90% of those infected become chronic carriers. • Chronic carriers have: • a 20% chance of developing cirrhosis • increased risk for developing hepatocellulalar carcinoma.

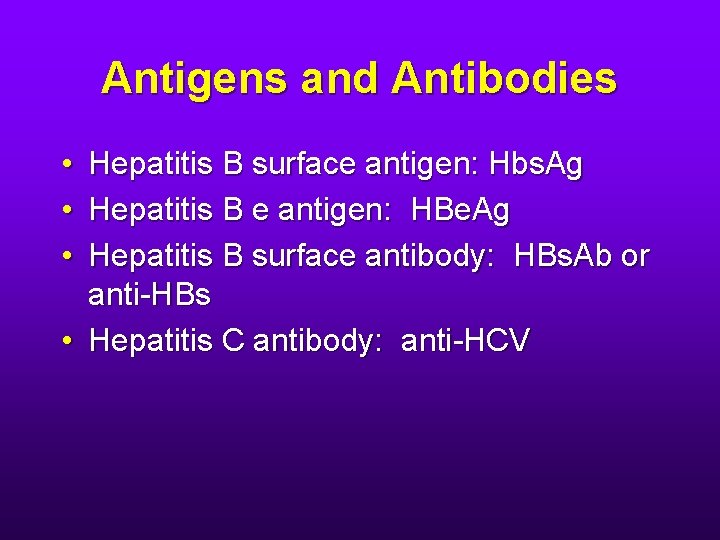
Antigens and Antibodies • Hepatitis B surface antigen: Hbs. Ag • Hepatitis B e antigen: HBe. Ag • Hepatitis B surface antibody: HBs. Ab or anti-HBs • Hepatitis C antibody: anti-HCV

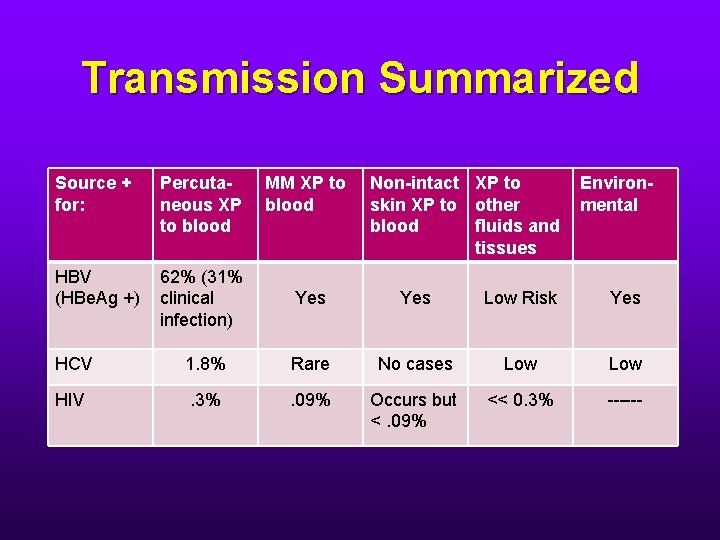
Occupational Transmission of HBV • Percutaneous: • Risk of infection depends on: • Degree of contact with blood • Hepatitis e antigen status of the source person. • When the source of the HBV-contaminated needle stick is HBe. Ag +, the risk of clinical hepatitis to the unvaccinated HCW is 22%31%. The risk of developing serologic evidence of HBV infection is 37%-62%

Occupational Transmission of HBV • Although percutaneous injuries are the most efficient modes of HBV infection, they account for only a minority of HBV infections among HCW’s. • Most infected HCW’s have not been able to recall an overt percutaneous injury although up to 1/3 can recall caring for a patient who was HBs. Ag +. • HBV survives in dried blood at room temperature on environmental surfaces for at least 1 week. • HBV infections might have resulted from mucosal exposures or exposures that inoculated HBV into breaks in the skin. • The potential for HBV transmission through contact with environmental surfaces has been demonstrated.

HBV Vaccine • HBs. Ab is protective (forever). • We can stimulate the body’s production of HBs. Ab by injecting HBs. Ag. • Injections at 0, 1 and 6 months. • If interrupted, just resume ASAP (no need to start over) • The last dose confers long term immunity. It must be given at least 2 months after the 2 nd dose in order to do this.

Documentation of Serologic Status • Every HCW who expects ongoing exposure to blood or blood products should know his/her serologic status. • Why? Because documenting surface antibody protection at any time following the vaccination series significantly streamlines post-exposure management for hepatitis B.

Documentation of Serologic Status • 90% of 40 year olds develop protective levels of surface antibody after vaccination. • 75% of 60 year olds do.

Documentation of Serologic Status • Testing for HBs. Ab should be performed 1 -2 months after completion of the 3 -dose vaccination series. • Persons who do not respond to the 1 st 3 -dose series should receive a 2 nd 3 -dose series. 3050% of persons who do not respond to the 1 st series respond to the 2 nd. • Revaccinated persons should be retested at the completion of the 2 nd vaccine series.

Documentation of Serologic Status • What about those of us who were vaccinated but never had a titer done? • In 50% of responders, the antibody titer declines over time and > 6 months after vaccination, can be undetectable or lower than what is usually considered protective. These people remain protected. • However, it is not possible to distinguish the responder whose level of antibody declined (but is still protected) from the non-responder (who is not protected).

Nonresponders • Should be checked for HBs. Ag. • If negative for HBs. Ag, they remain susceptible to HBV infection and need to be treated as such upon exposure.

Boosters • Not necessary • Periodic serologic testing to monitor antibody concentrations after a person has been shown to have a protective level of HBs. Ab is not recommended.

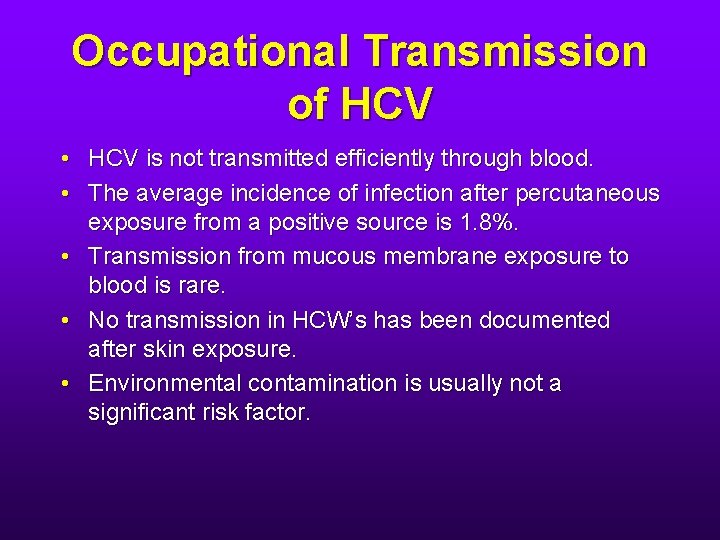
Occupational Transmission of HCV • HCV is not transmitted efficiently through blood. • The average incidence of infection after percutaneous exposure from a positive source is 1. 8%. • Transmission from mucous membrane exposure to blood is rare. • No transmission in HCW’s has been documented after skin exposure. • Environmental contamination is usually not a significant risk factor.

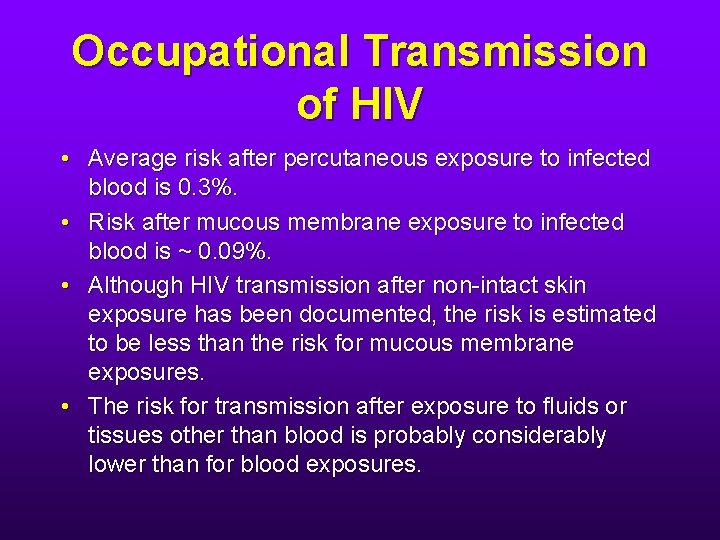
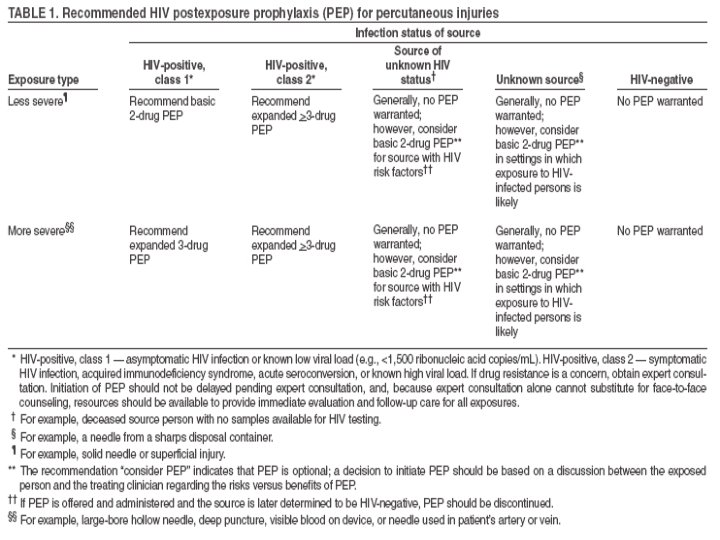
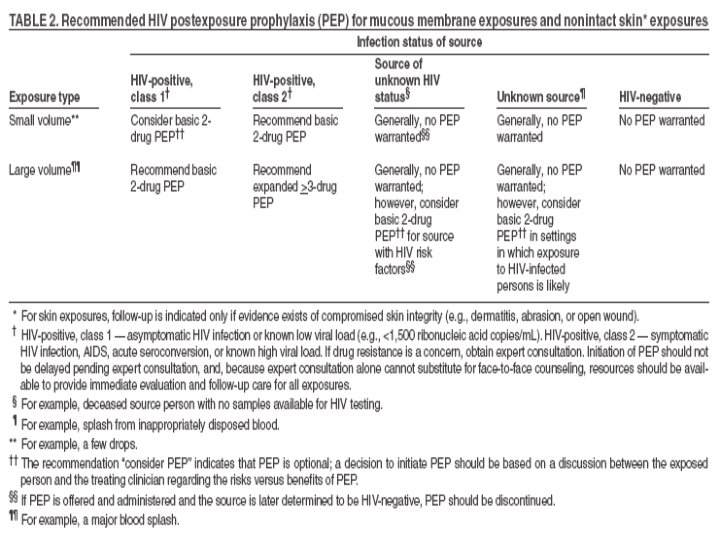
Occupational Transmission of HIV • Average risk after percutaneous exposure to infected blood is 0. 3%. • Risk after mucous membrane exposure to infected blood is ~ 0. 09%. • Although HIV transmission after non-intact skin exposure has been documented, the risk is estimated to be less than the risk for mucous membrane exposures. • The risk for transmission after exposure to fluids or tissues other than blood is probably considerably lower than for blood exposures.

Transmission Summarized Source + for: Percutaneous XP to blood MM XP to blood Non-intact XP to skin XP to other blood fluids and tissues Environmental HBV (HBe. Ag +) 62% (31% clinical infection) Yes Low Risk Yes HCV 1. 8% Rare No cases Low HIV . 3% . 09% Occurs but <. 09% << 0. 3% ------

What Happens After Exposure? • • • Treatment of Exposure Site: • Wounds and skin sites that have been in contact with blood or body fluids should be washed with soap and water. • Mucous membranes should be flushed with water. • No evidence exists that using antiseptics for wound care reduces the risk of transmission but their use is not contraindicated. • Do not: • Squeeze the wound • Apply caustic agents like bleach to the wound • Inject anything into the wound Get medical care immediately after treating the site! Notify your supervisor or infection control officer protocol.

Why the Rush? • Because PEP (for HIV) has to be started ASAP! • Animal studies suggest that PEP probably is substantially less effective when started more than 24 -36 hours postexposure. • In humans, the interval after which no benefit is gained from PEP is undefined. Therefore, PEP should be started even when the interval since exposure exceeds 36 hours. If the risk of transmission is very high, PEP can be started 1 week after exposure.

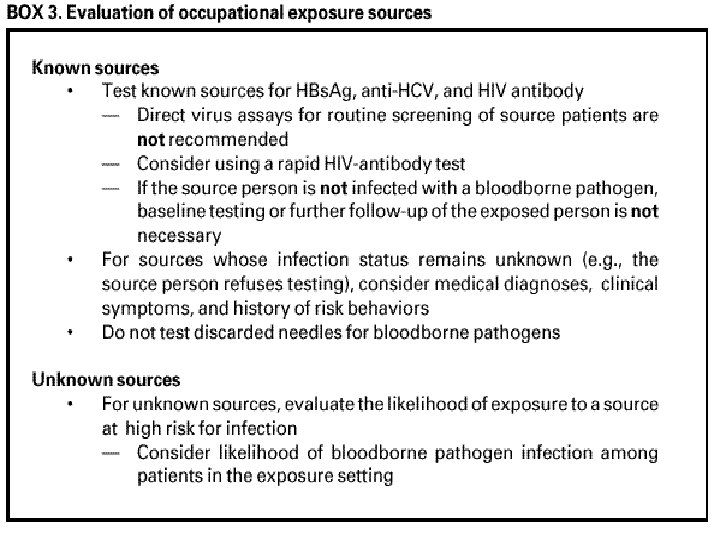
Evaluation of the Exposure • Type of exposure: • Percutaneous: For HCV and HIV, exposure to a blood-filled hollow needle or visibly bloody device suggests a higher risk exposure than exposure to a needle that was most likely used for giving an injection. • Mucous membrane exposure • Non-intact skin exposure • Bite


If the Source Person Is Known to Be HIV + • Available information about his stage of infection. • CD 4+ T-cell count • Viral load • Current and previous antiretroviral therapy • Results of resistance testing.

HIV Testing of the Source is Negative • If the source person is HIV negative and has no clinical evidence of AIDS or symptoms of HIV infection, no further testing of the person is indicated. • The likelihood of the source person being in the “window period” of HIV infection in the absence of symptoms of acute retroviral syndrome is extremely small.

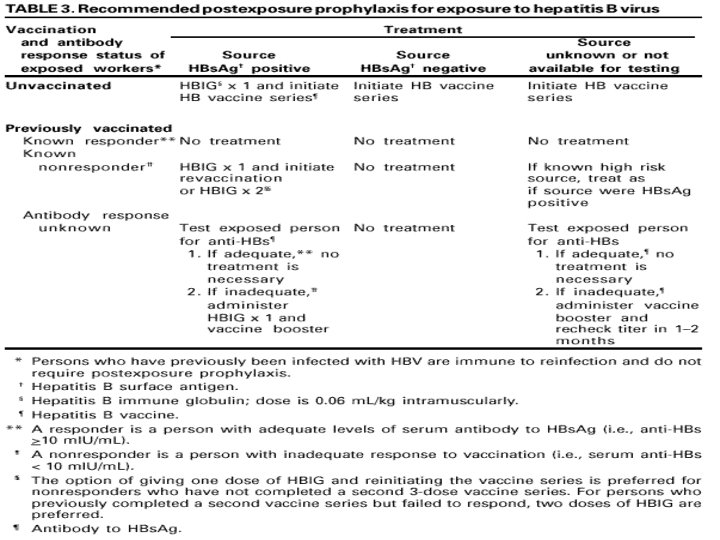
What Blood Tests Are You Going to Have? • HBV: You are only going to have a blood test for HBs. Ab if your antibody response to the vaccine is unknown. • If you have already had hepatitis B or if you are a known responder to the vaccine or a known nonresponder to the vaccine, you do not have to be tested. If you have never been vaccinated, you aren’t going to be tested. You are going to get HBIG and start the hepatitis B vaccine series.

What Blood Tests Are You Going to Have? • Hepatitis C: Baseline testing for anti-HCV and ALT. • HIV: If the source person is negative, you don’t have to be tested. However, serologic testing should be made available to all HCW’s who are concerned that they might have been exposed.

What Blood Tests Are You Going to Have? • If PEP for HIV is being considered, you will have a CBC, tests of kidney and liver function and possibly a glucose. (Protease inhibitors can cause diabetes, hyperglycemia and diabetic ketoacidosis. ) • You might also have a baseline urinalysis (indinavir can cause kidney stones. )

PEP: HBIG • HBIG is prepared from human plasma known to contain a high titer of anti-HBs. The plasma from which HBIG is prepared is screened for HBs. Ag and antibodies to HIV and HCV. • Since 1999, all products available in the US have been manufactured using methods that inactivate HCV and other viruses. • No evidence exists that HBV, HCV or HIV have ever been transmitted by HBIG commercially available in the US.

HBIG • When HBIG is indicated, it should be administered ASAP after the exposure (preferably within 24 hours). • The effectiveness of HBIG when administered > 7 days after exposure is unknown. • The dose is 0. 06 ml/kg IM (4. 2 ml for a 70 kg man)

HBIG Side Effects • Local pain and tenderness at the injection site • Allergic reactions: • • • Hives Angioedema Anaphylaxis

PEP: Hepatitis B Vaccine • When hepatitis B vaccine is indicated, it should also be administered ASAP (and preferably within 24 hours).




What Behavioral Changes Are You Going to Have to Make? • Exposure to HBV or HCV infected blood: • Refrain from donating blood, plasma, organs, tissue or semen. • The exposed person does not need to modify sexual practices or refrain from becoming pregnant. • If an exposed woman is breast feeding, she does not need to discontinue.

What Behavioral Changes Are You Going to Have to Make? • HIV Exposure: Especially during the 1 st 6 -12 weeks post – exposure when most HIV-infected persons are expected to seroconvert: • Sexual abstinence or use condoms to prevent sexual transmission and avoid pregnancy. • Don’t donate blood plasma, organs, tissue or semen. • HIV can be transmitted through breast milk – consider discontinuation of breast feeding, especially for high risk exposures. • Some PEP drugs pass into breast milk.

How Is My Job Going to Be Affected by My Exposure? • HBV or HCV exposure: No modifications necessary. • HIV exposure: No modifications necessary. • If you become infected with HIV or HBV, your patient care responsibilities might be affected. • There are no recommendations regarding restricting the professional activities of HCP’s with HCV infection.

What Next? • You should be seen again 72 hours after exposure. • More information about the exposure source should be available by then. • You will undoubtedly have questions that will need to be answered. • Counseling continues: You will be asked to seek medical evaluation for any acute illness that occurs during the ensuing follow up period. Such an illness, particularly if characterized by fever, rash, myalgia, fatigue, malaise or lymphadenopathy, might be indicative of acute HIV infection or a drug reaction.

And Then? • If PEP for HIV was started, you will be seen 2 weeks afterward. • Baseline lab tests (CBC, kidney function, liver function, glucose and urinalysis) will be repeated.

How Long Do I Need to Take PEP? • The optimal duration of PEP is unknown. Because 4 weeks of ZDV appeared protective in occupational and animal studies, PEP probably should be administered for 4 weeks, if tolerated.

Why Is PEP Discontinued? Side Effects, Side Effects • 50% of HCW’s experience nausea, malaise, headache or anorexia while taking PEP. • 17 -47% of HCW’s stop taking PEP because of side effects. • More side effects with the 3 -drug regimen than the 2 -drug regimen.

Nausea and Vomiting • The most common side effect (26. 5% of HCW’s taking PEP. • Can often be managed with anti-motility and anti-emetic agents without changing the regimen. • Administering a lower dose of drug more frequently throughout the day might facilitate adherence.

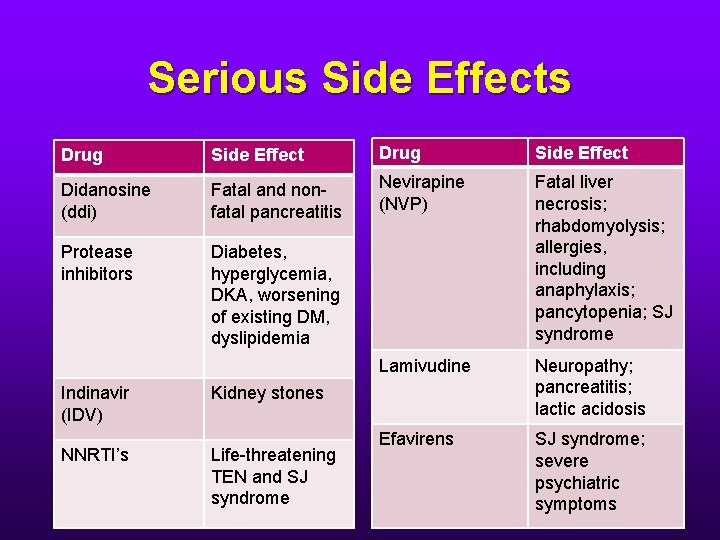
Serious Side Effects Drug Side Effect Didanosine (ddi) Fatal and nonfatal pancreatitis Nevirapine (NVP) Protease inhibitors Diabetes, hyperglycemia, DKA, worsening of existing DM, dyslipidemia Fatal liver necrosis; rhabdomyolysis; allergies, including anaphylaxis; pancytopenia; SJ syndrome Lamivudine Neuropathy; pancreatitis; lactic acidosis Efavirens SJ syndrome; severe psychiatric symptoms Indinavir (IDV) NNRTI’s Kidney stones Life-threatening TEN and SJ syndrome

Serious Drug Interactions • All of these drugs have the potential for serious drug interactions. Examples: • They decrease the clearance of statins, predisposing to the development of rhabdomyolysis. • PI’s can accelerate the clearance of OC’s. • St. John’s Wort increases clearance of PI’s. • Garlic lowers the level of saquinavir. • These drugs can interfere with each other.

Resistance • When the source person’s virus is known or suspected to be resistant to one or more of the drugs considered for the PEP regimen, the selection of drugs to which the source person’s virus is unlikely to be resistant is recommended. • Resistance testing of the source person’s virus at the time of exposure is not recommended. Results will come back too late to be of use (1 -2 weeks). • Rather, information about the source person’s medication history, clinical response to medications, CD 4+ counts, viral load measurements and current disease stage should be used. • If this information is not readily available, start PEP and revise later: one good reason for the 72 hour follow up.

Resistance • Occupational transmission of drugresistant HIV strains despite PEP with combination drug regimens has been reported • Luckily, not often. As of 09/30/05, only six cases of occupational HIV seroconversion despite combination PEP have been reported.

Follow Up for Exposures to HCV • For the person exposed to an HCVpositive source: • There is no PEP. • After baseline testing for anti-HCV and ALT, repeat testing should be performed in 4 -6 months. • If an earlier diagnosis of HCV infection is desired, testing for HCV RNA may be performed at 4 -6 weeks.

Follow Up for Exposure to HIV • HIV testing at baseline and then at 6 weeks, 12 weeks and 6 months post-exposure. • If you become infected with HCV after exposure to a source co-infected with HIV and HCV, another test in 12 months is recommended. • We don’t know if you should receive a test in 12 months if you are exposed to a co-infected source and you don’t develop HCV or if you are immunosuppressed.

Use of Direct Virus Assays • • • HIV p 24, HIV RNA Not recommended These can detect HIV a few days earlier than the routinely used assay but the infrequency of occupational seroconversion and increased costs of these tests do not warrant their routine use in this setting. • Relatively high false positive rate.

• HIV testing should be performed on any exposed person who has an illness compatible with an acute retroviral syndrome, regardless of the interval since exposure. • Fever, rash, myalgia, fatigue, malaise or lymphadenopathy.

Question #1 Which of the following exposures pose a risk for bloodborne pathogen infection? 1. A nurse sustains a needle stick while drawing up insulin to administer to a patient with diabetes. 2. A lab worker is splashed in the eye with urine from a patient with HIV. 3. An operating room technician with chapped and abraded hands notices blood under his/her gloves after assisting in a surgery on a patient with hepatitis C infection. 4. While cleaning the bathroom, a housekeeper’s intact skin has contact with feces.

Question 2 Which lab tests should be completed for the exposed person to determine his/her susceptibility to a bloodborne pathogen infection? 1. 2. 3. 4. 5. HBs. Ag HIV p 24 antigen HCV RNA All of the above None of the above

Question 3 Following a percutaneous exposure to HCV-infected blood what action(s) is/are recommended? 1. Test the exposed person for antibody to HCV and ALT at the time of exposure and 4 -6 month later. 2. Administer one dose of immune globulin within 7 days of the exposure. 3. Immediately start PEP with interferon and ribavirin. 4. All of the above. 5. None of the above.

Question 4 After completing the initial 3 -dose vaccine series against HBV, HCP’s who will have contact with patients or blood and are at ongoing risk for percutaneous injuries should have anti-HBs testing completed: 1. 2. 3. 4. 5. Every year. After any blood exposure. 1 -2 months after the completion of the vaccine series. All of the above None of the above.

Question 5 The type of occupational exposure to HIVinfected blood that poses the greatest risk for infection transmission is: 1. A percutaneous injury. 2. A mucous membrane injury 3. A bite 4. Skin contact with HIV-infected blood.

Question 6 For which of the following exposures would the use of HIV PEP be recommended? 1. A housekeeper sustains a percutaneous injury while emptying a needle box on a pediatric ward with no known cases of HIV infection. 2. A nurse has a urine splash to the eye while emptying an AIDS patient’s urinal. 3. A resident, after assisting with an emergency insertion of a central venous line into an HIV-infected patient, notices a small tear in his/her glove but does not observe any blood on his/her skin. 4. A phlebotomist sustains a percutaneous injury while performing phlebotomy on an HIV-infected patient. 5. All of the above.

Question 7 Following an exposure to a bloodborne pathogen, what information would be included as part of the post-exposure counseling? (Indicate all that apply. ) 1. HCP exposed to HBV and HCV do not need to take any special precautions to prevent secondary transmission during the follow up period. 2. Modifying an exposed person’s patient care responsibilities is not necessary to prevent transmission to patients after an exposure to HBV, HCV or HIV. 3. HCP who have an HIV exposure for which PEP is not recommended should be informed that the potential side effects and toxicity of taking PEP outweigh the risk of transmission posed by the type of exposure. 4. HCP should seek medical evaluation for a any acute illness that occurs during the follow-up period.