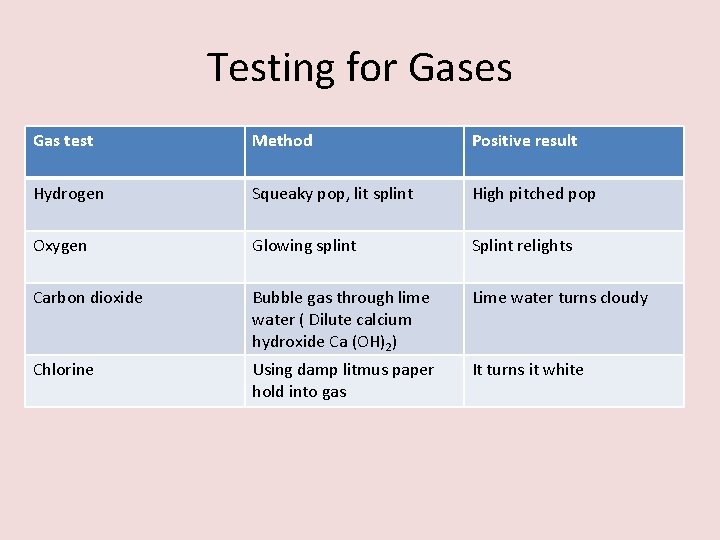
Testing for Gases Gas test Method Positive result












- Slides: 16

Testing for Gases Gas test Method Positive result Hydrogen Squeaky pop, lit splint High pitched pop Oxygen Glowing splint Splint relights Carbon dioxide Bubble gas through lime water ( Dilute calcium hydroxide Ca (OH)2) Lime water turns cloudy Chlorine Using damp litmus paper hold into gas It turns it white

Gas tests link • https: //www. youtube. com/watch? v=j. Vk. GKurt ai. E • https: //www. youtube. com/watch? v=f. CZztw. J m. Al 0

• • Safety precautions: Use small quantities How flammable the gas Do you need to use a fume cupboard Is it an irritant Wear googles tongs

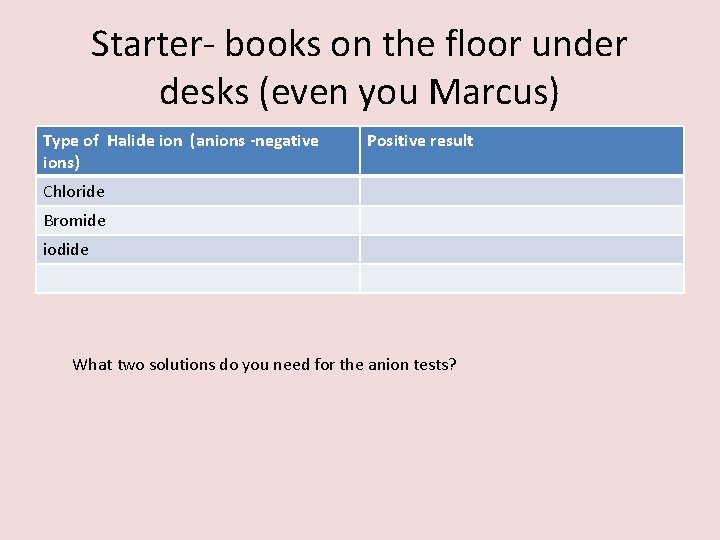
Identifying ions Type of Halide ion (anions -negative ions) Positive result Chloride White precipitate Bromide Cream precipitate iodide Yellow precipitate Nitric acid is added to the test as it is a catalyst Each sodium salt was added to distilled water ( No other ions to interfere with the test) The test solution Silver Nitrate The precipitates are silver chloride, silver bromide or silver iodide – THESE ARE ALL INSOLUBLE

Starter- books on the floor under desks (even you Marcus) Positive result • On scrap paper complete the following table: Type of Halide ion (anions -negative ions) Chloride Bromide iodide What two solutions do you need for the anion tests?

Outcomes All to recall that flame tests can be used to identify some metal ions (cations). Lithium, sodium, potassium, calcium and copper compounds to explain that if a sample containing a mixture of ions is used some flame colours can be masked • Most to be able to interpret an instrumental result given appropriate data in chart or tabular form, when accompanied by a reference set in the same form, limited to flame emission spectroscopy • Some To explain how flame spectroscopy actually works

Flame test for metals Metal Colour of flame Lithium Crimson flame (red) Sodium Yellow Flame Potassium Lilac Flame Calcium Orange red flame Copper Green flame Which splint is which type of metal?

So what would you see if you had more than one metal present in solution?

Colour masking • If there is a combination of metals present in a solution the colours may mask each others. Eg the most vibrant colour will over shadow others so flame test by itself are not the most effective analytical tool

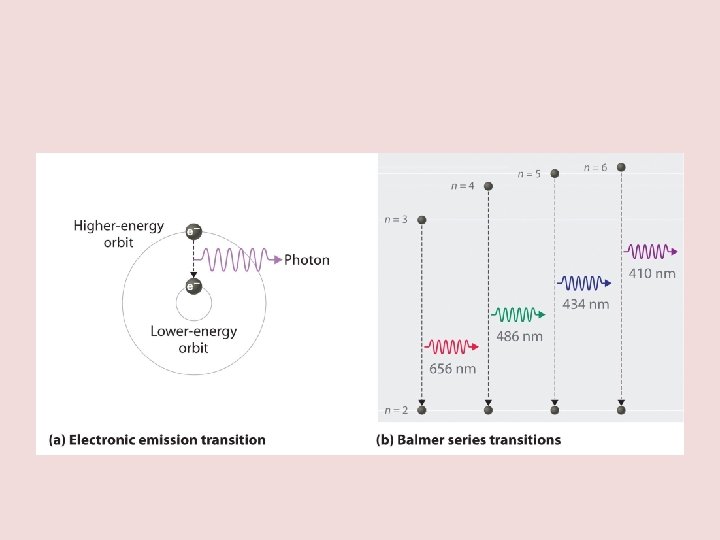
Flame Emission spectroscopy • All ions have a different electron configuration on their shells • When you heat ions up the electrons get excited and swap shells or energy level • When the electron drop back into place the energy is transferred into light • Different electrons combinations will therefore emit different patterns of light a bit like a fingerprint


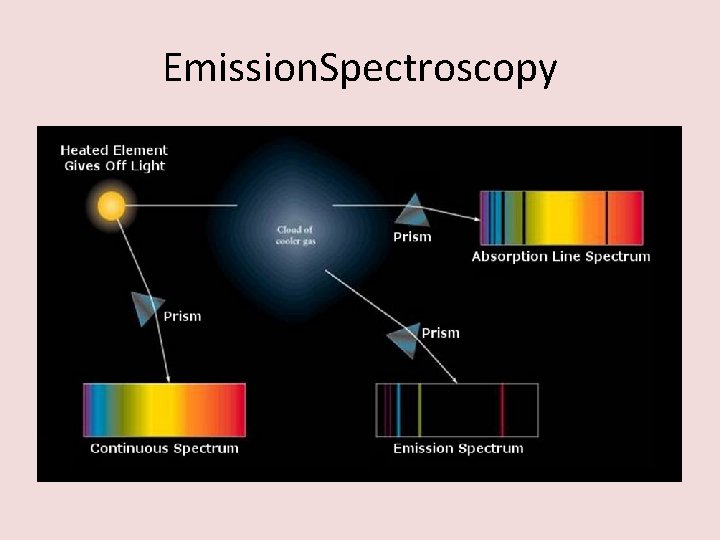
Emission. Spectroscopy

How to read an spectroscopy graph

Now have a go at the exam questions!

Cation – metal precipitate tests Metal Ion Colour of the Precipitate Ionic precipitation formula Calcium Ca 2+ White Ca(OH)2(s) Copper Cu 2+ Blue Cu(OH)2(s) Iron Fe 2+ Green Fe(OH)2(s) Iron Fe 3+ Brown Fe(OH)3(s) Aluminium Al 3+ White and then clear Al(OH)3(s) Magnesium Mg 2+ White Mg(OH)2(s) This is a displacement reaction with sodium hydroxide Metal hydroxides are insoluble so they form solid precipitate

Indicators Type of indicator Colour change Universal Acids are red 1 -6 Neutral green 7 Alkali are blue purple 8 -14 Acids red Neutral Red Alkali Blue Acid red Neutral blue Alkali blue Red litmus Blue Litmus