Testing for Equivalence or Non inferiority Level Intermediate
















- Slides: 18

Testing for Equivalence or Non -inferiority Level : Intermediate Version No: 1 Version Date: June 2013 Nurun Nisa de Souza, MD, MPH Associate Duke-NUS Graduate Medical School

Disclaimer/Liability • The information provided in the VAP is made available in good faith and is derived from sources believed to be reliable and accurate at the time of release. • The materials presented on the VAP may include links to external Internet sites. These external information sources are outside the control of Duke-NUS. The user of the Internet links is responsible for making his or her own decision about the accuracy, reliability and correctness of the information found. • In no event shall Duke-NUS be liable for any indirect, special, incidental, or consequential damages arising out of any use of reliance of any information contained in the VAP. Nor does Duke-NUS assume any responsibility for failure or delay in updating or removing the information contained in the VAP. • Moreover, information provided on the VAP does not constitute medical advice or treatment nor should it be considered as a replacement of the patient/physician relationship or a physician’s professional judgment. Duke. NUS expressly disclaims all liability for treatment, diagnosis, decisions and actions taken or not taken in reliance upon information contained in the VAP. This work is licensed under a Creative Commons Attribution-Non. Commercial-No. Derivs 3. 0 Unported License To view a copy of this license, visit [http: //creativecommons. org/licenses/by-nc-nd/3. 0/]

Financial Disclosures (past 3 years) • No Disclosures

Learning Objectives At the end of this presentation you will be able to: • Recognize equivalence and non-inferiority study designs and understand why they are done • Describe the assumptions behind such trial designs • Interpret equivalence and non-inferiority using 95% confidence intervals and hypothesis testing

Outline • Equivalence vs non-inferiority • The rationale • Equivalence trials • Non-inferiority trials • Summary

Equivalence vs Non-inferiority • Equivalence: – Goal: To determine whether the outcome of two treatments is not too different from one another within an acceptable range – Answers the question: ‘Does test drug B give a similar, or close, clinical outcome as standard drug A? ’ • Non-inferiority: – Goal: To demonstrate that a new treatment is not worse than standard treatment by an acceptable margin – Answers the question: Is test drug B not worse than standard drug A?

Equivalence vs Non-inferiority (con’t) Requirements: • Understand the drug action • Test treatment works • Test treatment must not give worse outcomes compared to standard treatment

Why equivalence or non-inferiority trials? • Well-established treatment for the condition already exists • New treatment is cheaper, safer, less invasive or easier to administer • Applicable in trials where giving patients a placebo is unethical

Equivalence

Equivalence trials • To prove equivalence, we wish to show that a new treatment does not differ from that of the standard treatment by more than a prespecified clinically significant amount. • Based on the assumption: H 0 = New treatment A is better than standard treatment B H 1 = New treatment A is gives a similar outcome as standard treatment B • Defined by a zone of equivalence, i. e. region between the upper and lower margins of an outcome of two treatments in that is scientifically or clinically acceptable as giving similar outcomes.

Equivalence trials (con’t) A B C D E Δ 1 Δ 2 New treatment worse New treatment better A. B. C. D. E. New treatment = standard treatment but data are inconclusive New treatment ≠ standard treatment

Equivalence trials (con’t) Testing for equivalence • Two treatments are equivalent only when all of the 95% CI lies within the zone of equivalence • Two cut-off margins needed to determine equivalence >> two hypotheses tested: 1. The mean value of the ratio is >Δ 1 and this increase is statistically significant, i. e. p < 0. 05. 2. The mean value of the ratio is < Δ 2 and this decrease is statistically significant, i. e. p < 0. 05. • Statistical significance (p-value) alone may not prove equivalence, rather it only allows one to reject H 0 in favor of H 1.

Non-inferiority trials

Non-inferiority trials • To prove non-inferiority, we wish to show that a new treatment is not worse than the standard treatment • Defined by a non-inferiority margin, below which the new treatment is deemed to be inferior to the standard • Similar concept as equivalence trials but all of the 95% CIs lie to the right of the inferiority margin • Based on the assumption: H 0 = New treatment is not inferior to the standard treatment H 1 = New treatment is inferior to the standard treatment

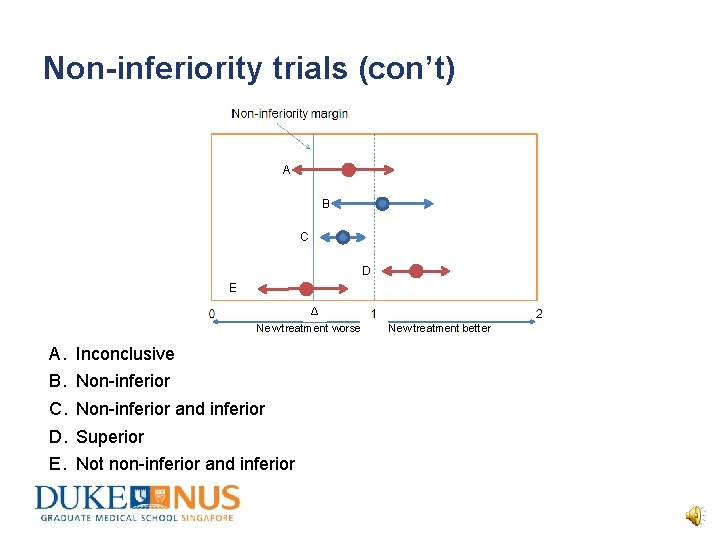
Non-inferiority trials (con’t) A B C D E Δ New treatment worse New treatment better A. B. C. D. E. Inconclusive Non-inferior and inferior Superior Not non-inferior and inferior

Non-inferiority trials (con’t) Testing for non-inferiority • As in equivalence testing, the 95% CI determines whether or not the treatment is non-inferior. • Since there is only one cut-off margin, i. e. non-inferiority margin, assuming that anything below Δ is inferior, the following holds true: – The mean value of the ratio is > Δ and this increase is statistically significant, i. e. p < 0. 05. • 95% CI relates to clinical significance while p-value relates to statistical significance, i. e. whether to accept or reject H 0.

Summary • Hypothesis testing does not conclude equivalence or non-inferiority; it only allows one to accept or reject H 0. • Before testing for equivalence or non-inferiority, be certain that standard treatment is superior to placebo. • Confidence intervals, and not point estimates, determine equivalence or non-inferiority.

Thank you www. duke-nus. edu. sg