Test 1 Lab Test 18 20 s Lab

Test 1 Lab Test • 18 -20 ? s • Lab format with follow up questions – – – Measure Observations PP, CP, PC, CC Inquiry Substances/mixtures Written Test • • 42 ? s MC (multiple choice) One free response All items from study guide
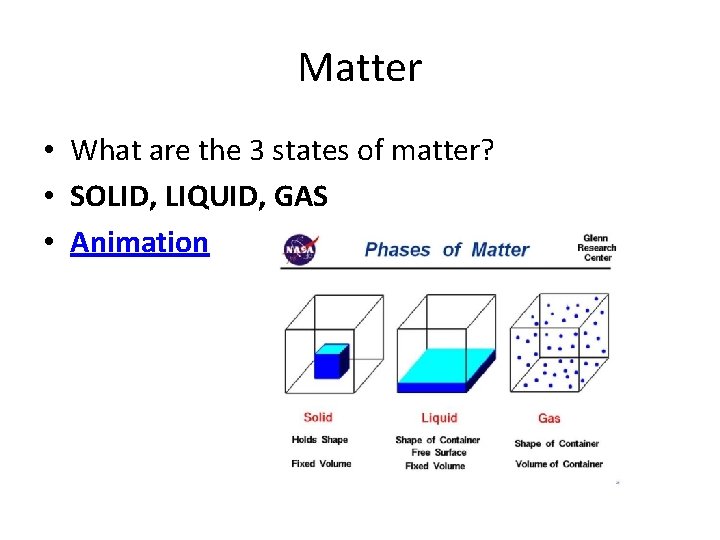
Matter • What are the 3 states of matter? • SOLID, LIQUID, GAS • Animation
• Pure substance Elements
Compounds • Compound = a pure chemical substance of 2 or more different elements that can only be separated into simpler substances by chemical reactions • Ex: H 2 O, CO 2, Sugar, Salt

Mixtures • Mixture = when 2 or more substances combine (physically) and both substance keep their individual properties • Ex: – flour & sugar – Salt water – Salad
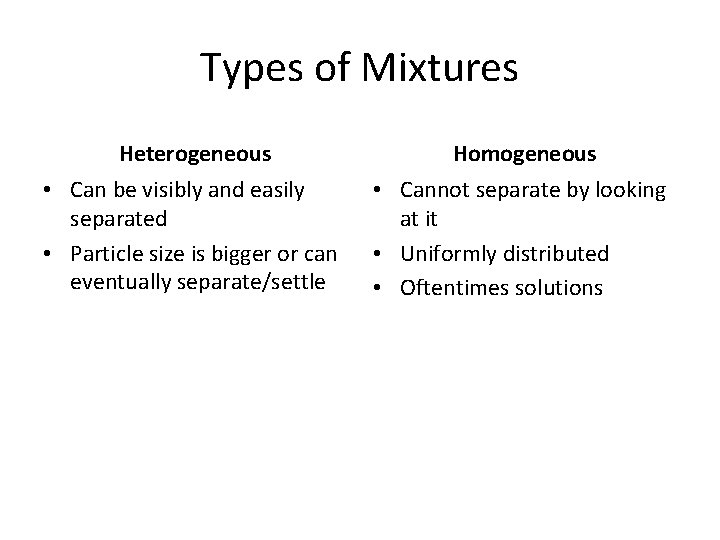
Types of Mixtures Heterogeneous Homogeneous • Can be visibly and easily separated • Particle size is bigger or can eventually separate/settle • Cannot separate by looking at it • Uniformly distributed • Oftentimes solutions

HE or HO • • • Vinegar Sand Air Sugar water Vinegar + oil Chex mix
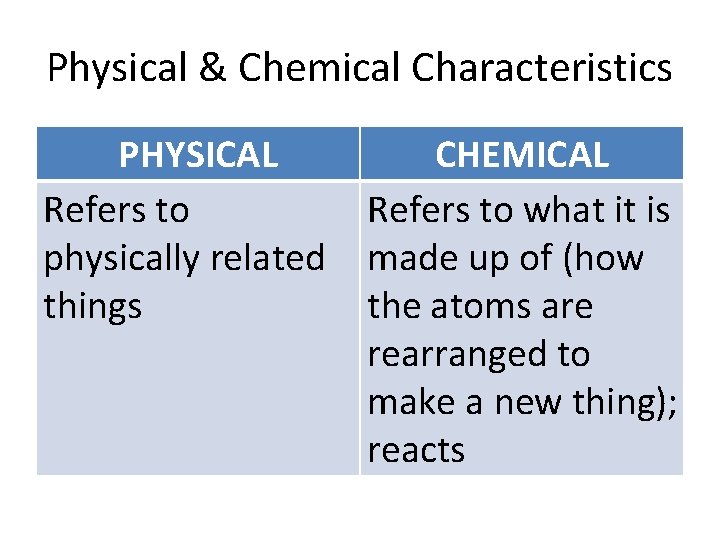
Physical & Chemical Characteristics PHYSICAL Refers to physically related things CHEMICAL Refers to what it is made up of (how the atoms are rearranged to make a new thing); reacts

Physical or Chemical • Physical = it looks different BUT the atoms are still the same you could reverse it • Chemical = may or may not look different BUT the atoms are rearranged differently you CAN’T reverse it * Unless you do another chemical reaction

Properties • • • What is a property? Describes, talks about it’s ability to… Can be physical or chemical Has it CHANGED yet? . . NO Does it describe it/have ability to change?
Examples of Properties • Front Desk: PHYSICAL PROPERTIES CHEMICAL PROPERTIES
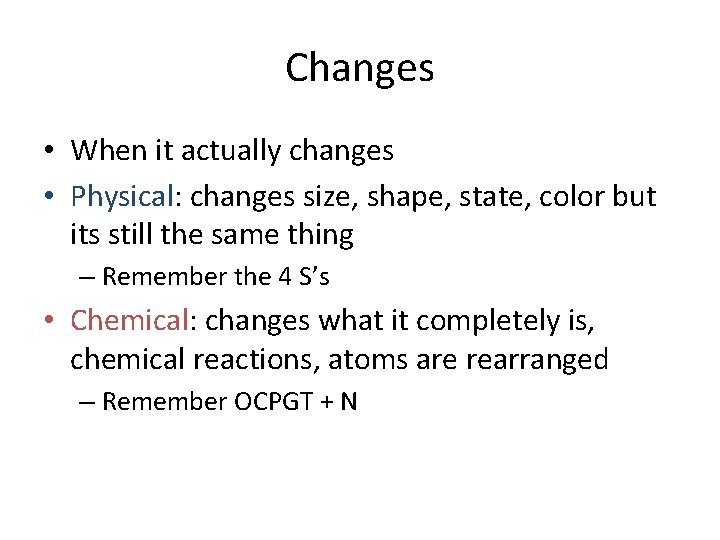
Changes • When it actually changes • Physical: changes size, shape, state, color but its still the same thing – Remember the 4 S’s • Chemical: changes what it completely is, chemical reactions, atoms are rearranged – Remember OCPGT + N

Examples of changes • • • Tearing paper Physical change Burning paper Chemical change Breaking wood Physical change Chemical change Campfire Mixing kool aid Physical change Cooking a cake Chemical change
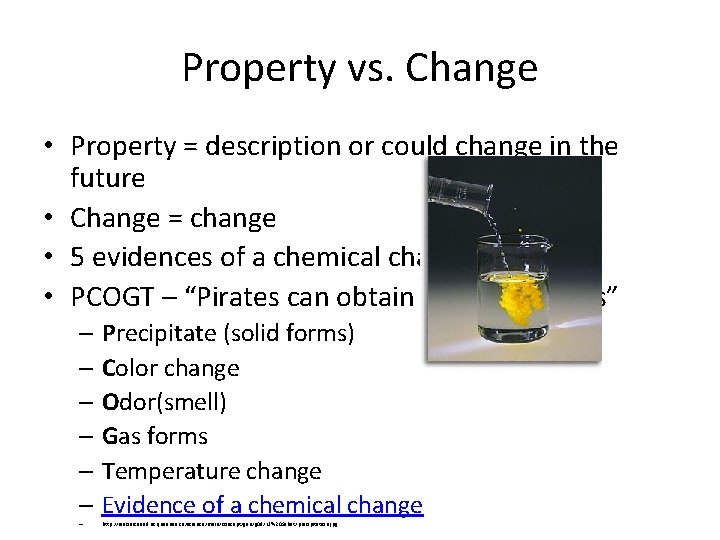
Property vs. Change • Property = description or could change in the future • Change = change • 5 evidences of a chemical change • PCOGT – “Pirates can obtain great treasures” – Precipitate (solid forms) – Color change – Odor(smell) – Gas forms – Temperature change – Evidence of a chemical change – http: //resources. educ. queensu. ca/science/main/concept/gen/g 09/N. %20 Sabet/precipitation. jpg

Property or Change? • • • Property Boiling point 2 different liquids mix bubbles Change Food coloring in water Property Burning an egg Change It has a yellow color Property It produced a smell Change
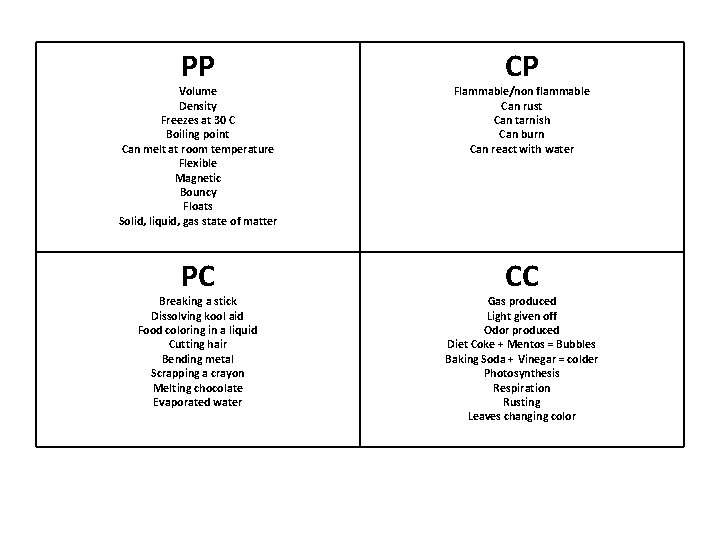
PP CP Volume Density Freezes at 30 C Boiling point Can melt at room temperature Flexible Magnetic Bouncy Floats Solid, liquid, gas state of matter Flammable/non flammable Can rust Can tarnish Can burn Can react with water PC CC Breaking a stick Dissolving kool aid Food coloring in a liquid Cutting hair Bending metal Scrapping a crayon Melting chocolate Evaporated water Gas produced Light given off Odor produced Diet Coke + Mentos = Bubbles Baking Soda + Vinegar = colder Photosynthesis Respiration Rusting Leaves changing color
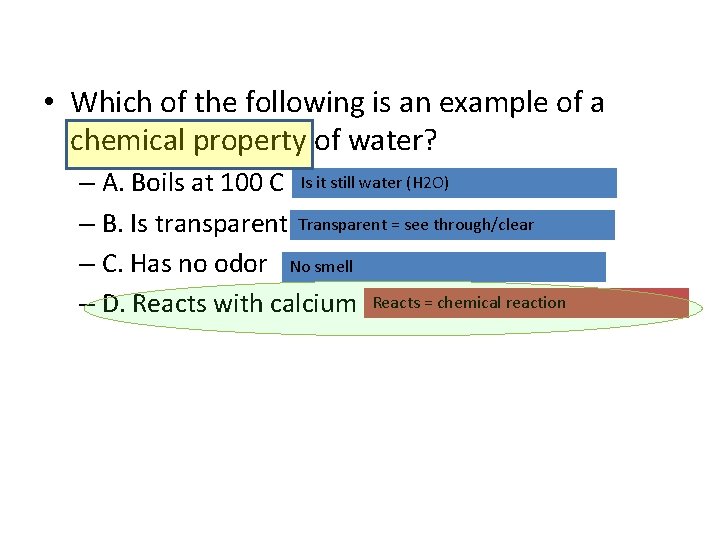
• Which of the following is an example of a chemical property of water? – A. Boils at 100 C Is it still water (H 2 O) – B. Is transparent Transparent = see through/clear – C. Has no odor No smell – D. Reacts with calcium Reacts = chemical reaction

• Which of the following is an example of a PHYSICAL PROPERTY physical property of hydrogen? It • A. Is less dense than air • B. Reacts with oxygen • C. Is highly flammable • D. Forms hydrochloric acid

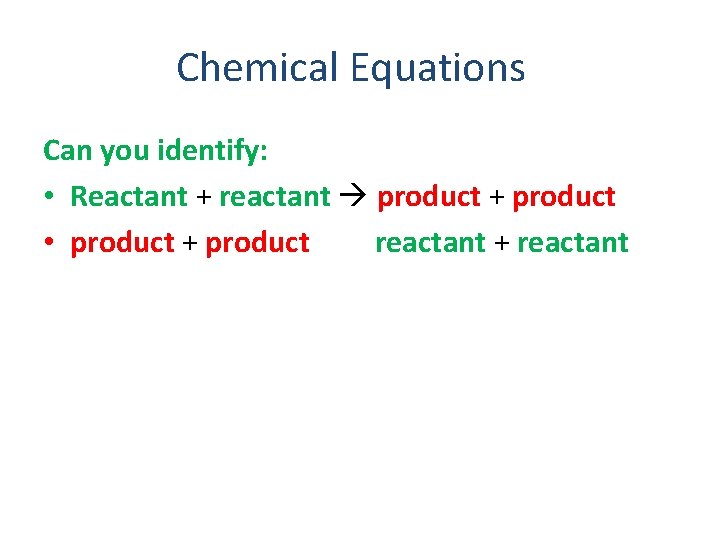
Chemical Equations Can you identify: • Reactant + reactant product + product • product + product reactant + reactant
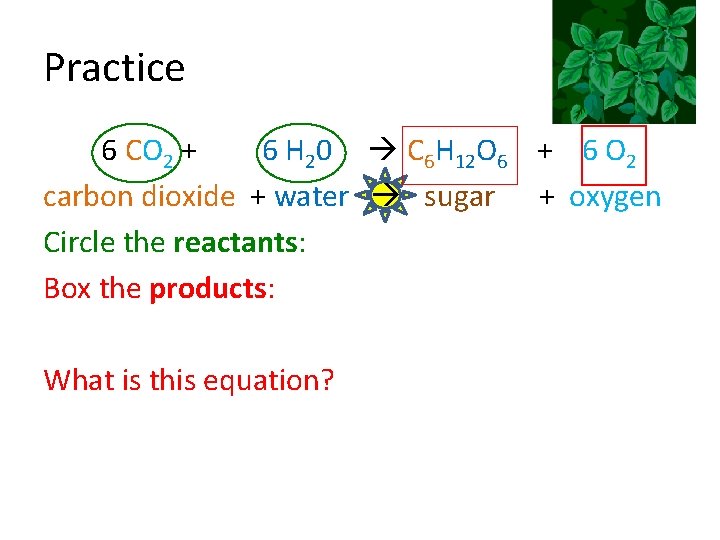
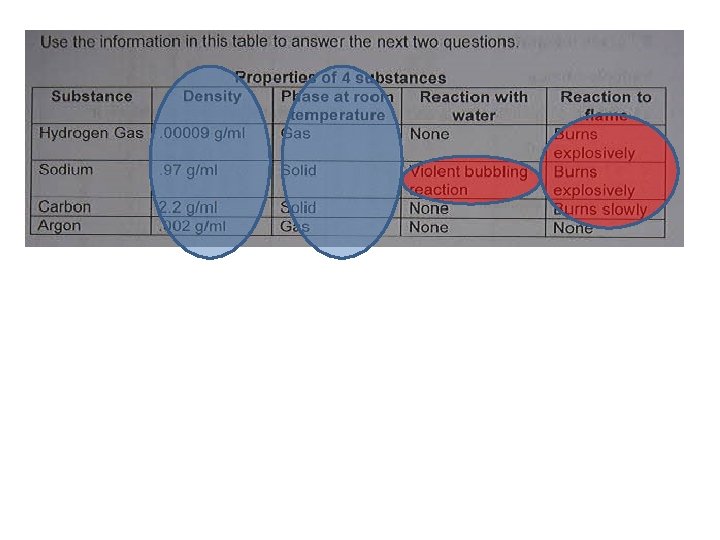
Practice 6 CO 2 + 6 H 20 C 6 H 12 O 6 + 6 O 2 carbon dioxide + water sugar + oxygen Circle the reactants: Box the products: What is this equation?
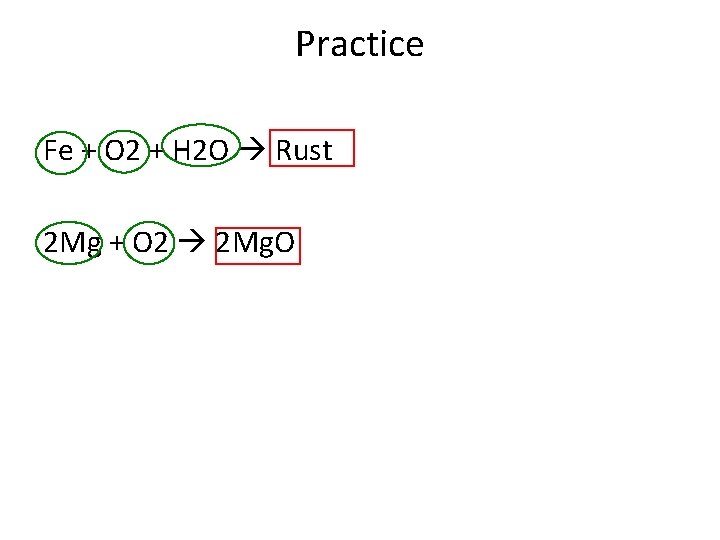
Practice Fe + O 2 + H 2 O Rust 2 Mg + O 2 2 Mg. O
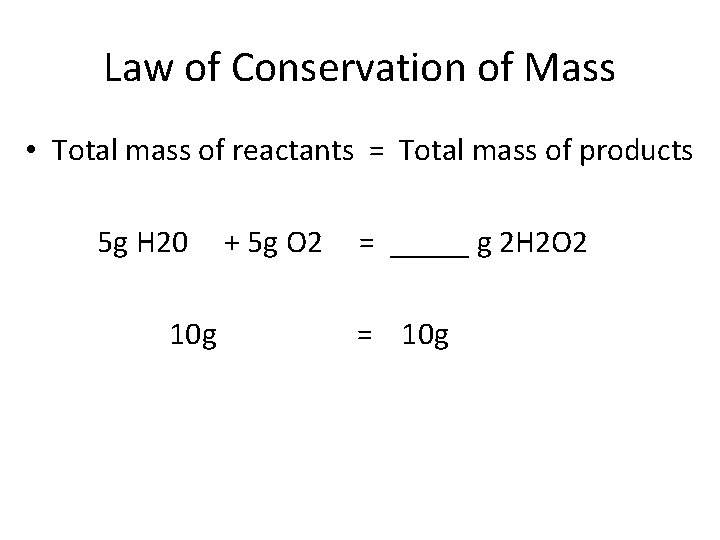
Law of Conservation of Mass • Total mass of reactants = Total mass of products 5 g H 20 10 g + 5 g O 2 = _____ g 2 H 2 O 2 = 10 g
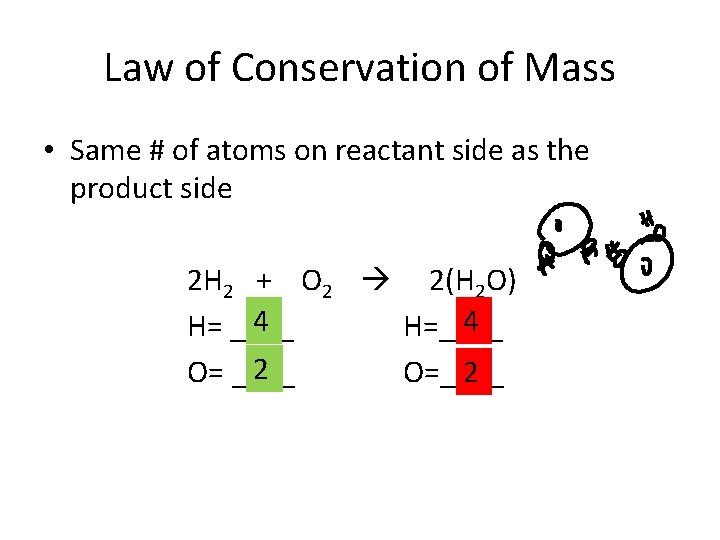
Law of Conservation of Mass • Same # of atoms on reactant side as the product side 2 H 2 + O 2 2(H 2 O) 4 4 H= ____ H=____ 2 2 O= ____ O=____
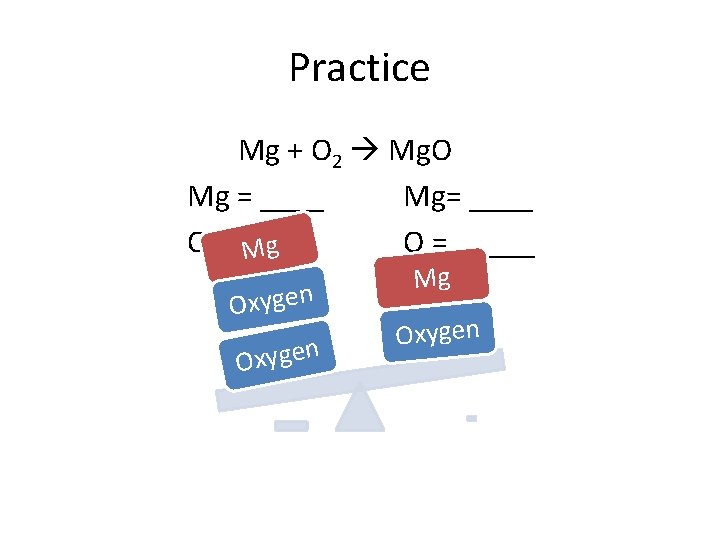
Practice Mg + O 2 Mg. O Mg = ____ Mg= ____ O = _____ Mg Oxygen

Inquiry • • IV DV Constants Control
- Slides: 27