Terpenoids Terpenes and terpenoids are the most important

- Slides: 15

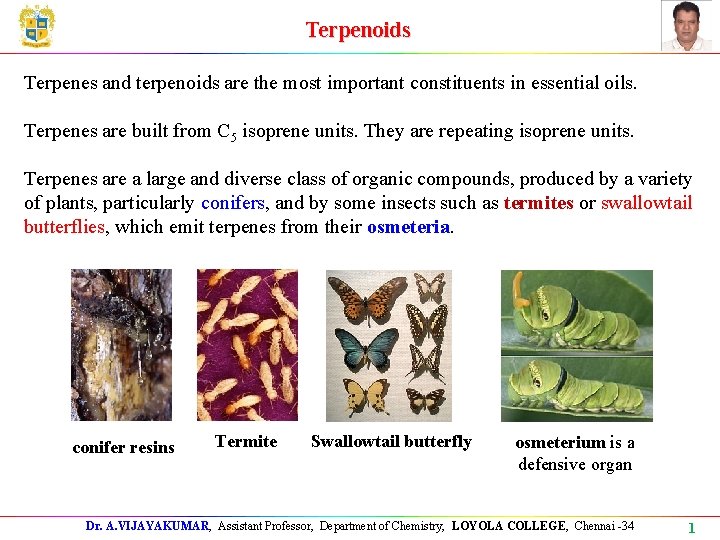
Terpenoids Terpenes and terpenoids are the most important constituents in essential oils. Terpenes are built from C 5 isoprene units. They are repeating isoprene units. Terpenes are a large and diverse class of organic compounds, produced by a variety of plants, particularly conifers, and by some insects such as termites or swallowtail butterflies, which emit terpenes from their osmeteria. conifer resins Termite Swallowtail butterfly osmeterium is a defensive organ Dr. A. VIJAYAKUMAR, Assistant Professor, Department of Chemistry, LOYOLA COLLEGE, Chennai -34 1

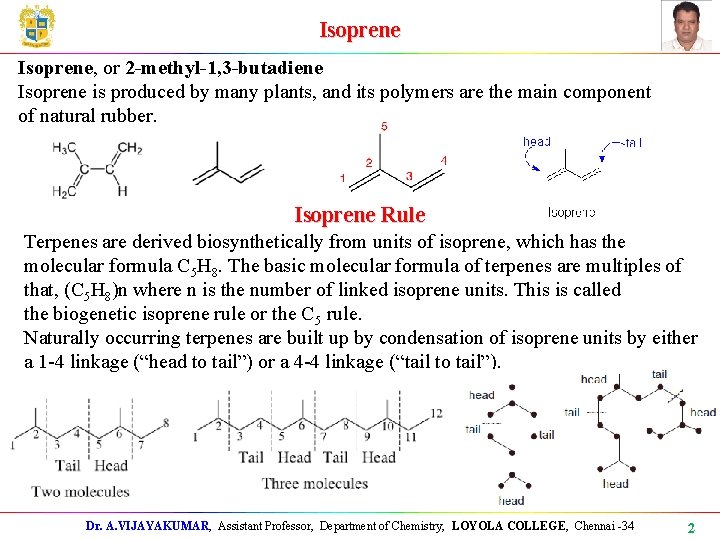
Isoprene, or 2 -methyl-1, 3 -butadiene Isoprene is produced by many plants, and its polymers are the main component of natural rubber. Isoprene Rule Terpenes are derived biosynthetically from units of isoprene, which has the molecular formula C 5 H 8. The basic molecular formula of terpenes are multiples of that, (C 5 H 8)n where n is the number of linked isoprene units. This is called the biogenetic isoprene rule or the C 5 rule. Naturally occurring terpenes are built up by condensation of isoprene units by either a 1 -4 linkage (“head to tail”) or a 4 -4 linkage (“tail to tail”). Dr. A. VIJAYAKUMAR, Assistant Professor, Department of Chemistry, LOYOLA COLLEGE, Chennai -34 2

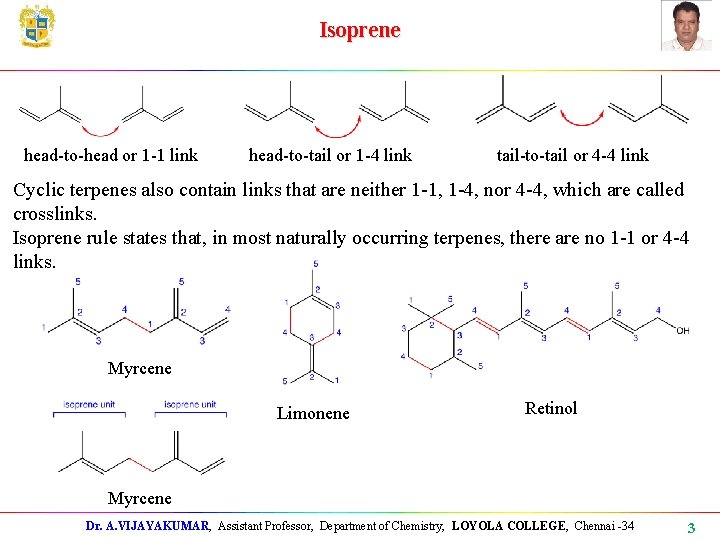
Isoprene head-to-head or 1 -1 link head-to-tail or 1 -4 link tail-to-tail or 4 -4 link Cyclic terpenes also contain links that are neither 1 -1, 1 -4, nor 4 -4, which are called crosslinks. Isoprene rule states that, in most naturally occurring terpenes, there are no 1 -1 or 4 -4 links. Myrcene Limonene Retinol Myrcene Dr. A. VIJAYAKUMAR, Assistant Professor, Department of Chemistry, LOYOLA COLLEGE, Chennai -34 3

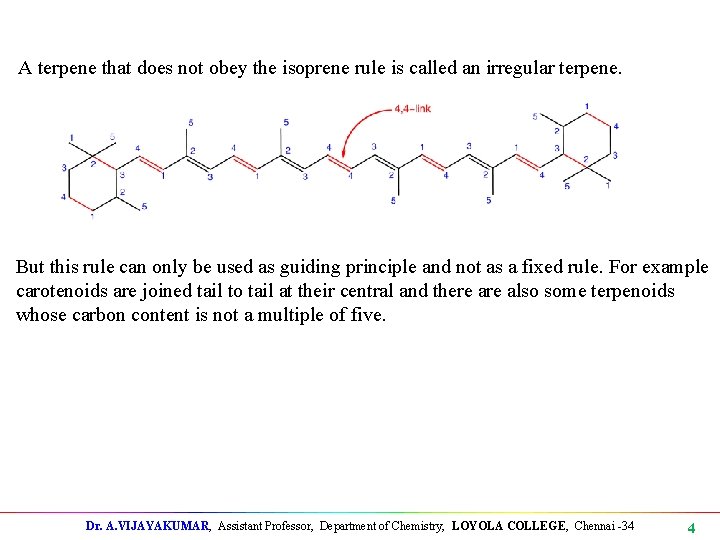
A terpene that does not obey the isoprene rule is called an irregular terpene. But this rule can only be used as guiding principle and not as a fixed rule. For example carotenoids are joined tail to tail at their central and there also some terpenoids whose carbon content is not a multiple of five. Dr. A. VIJAYAKUMAR, Assistant Professor, Department of Chemistry, LOYOLA COLLEGE, Chennai -34 4

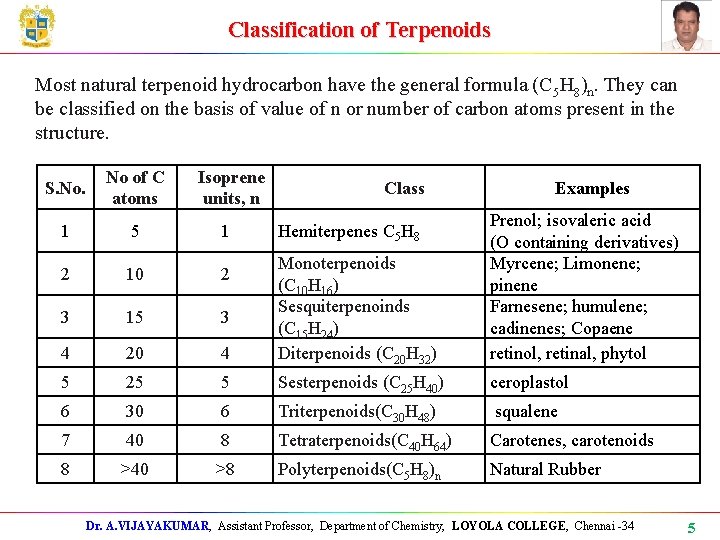
Classification of Terpenoids Most natural terpenoid hydrocarbon have the general formula (C 5 H 8)n. They can be classified on the basis of value of n or number of carbon atoms present in the structure. S. No. No of C atoms Isoprene units, n 1 5 1 2 10 2 3 15 3 4 20 5 Class Examples 4 Monoterpenoids (C 10 H 16) Sesquiterpenoinds (C 15 H 24) Diterpenoids (C 20 H 32) Prenol; isovaleric acid (O containing derivatives) Myrcene; Limonene; pinene Farnesene; humulene; cadinenes; Copaene retinol, retinal, phytol 25 5 Sesterpenoids (C 25 H 40) ceroplastol 6 30 6 Triterpenoids(C 30 H 48) squalene 7 40 8 Tetraterpenoids(C 40 H 64) Carotenes, carotenoids 8 >40 >8 Polyterpenoids(C 5 H 8)n Natural Rubber Hemiterpenes C 5 H 8 Dr. A. VIJAYAKUMAR, Assistant Professor, Department of Chemistry, LOYOLA COLLEGE, Chennai -34 5

Each class can be further subdivided into subclasses according to the number of rings present in the structure. • Acyclic Terpenoids: They contain open structure. • Monocyclic Terpenoids: They contain one ring in the structure. • Bicyclic Terpenoids: They contain two rings in the structure. • Tricyclic Terpenoids: They contain three rings in the structure. • Tetracyclic Terpenoids: They contain four rings in the structure. Some examples of mono, sesqui and diterpenoids: I. Mono Terpenoids: Acyclic Monoterpenoids Monocyclic monoterpenoids Dr. A. VIJAYAKUMAR, Assistant Professor, Department of Chemistry, LOYOLA COLLEGE, Chennai -34 6

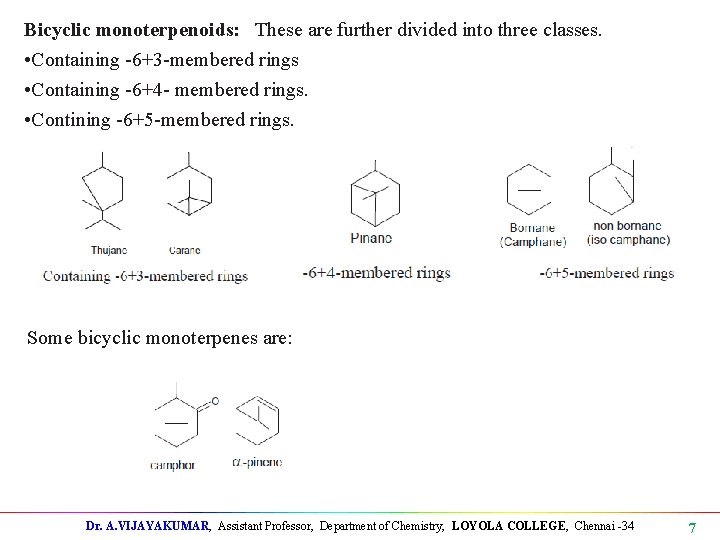
Bicyclic monoterpenoids: These are further divided into three classes. • Containing -6+3 -membered rings • Containing -6+4 - membered rings. • Contining -6+5 -membered rings. Some bicyclic monoterpenes are: Dr. A. VIJAYAKUMAR, Assistant Professor, Department of Chemistry, LOYOLA COLLEGE, Chennai -34 7

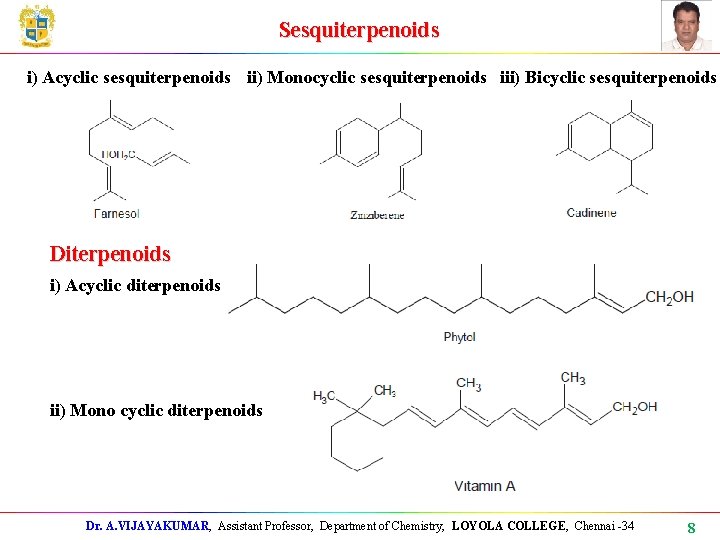
Sesquiterpenoids i) Acyclic sesquiterpenoids ii) Monocyclic sesquiterpenoids iii) Bicyclic sesquiterpenoids Diterpenoids i) Acyclic diterpenoids ii) Mono cyclic diterpenoids Dr. A. VIJAYAKUMAR, Assistant Professor, Department of Chemistry, LOYOLA COLLEGE, Chennai -34 8

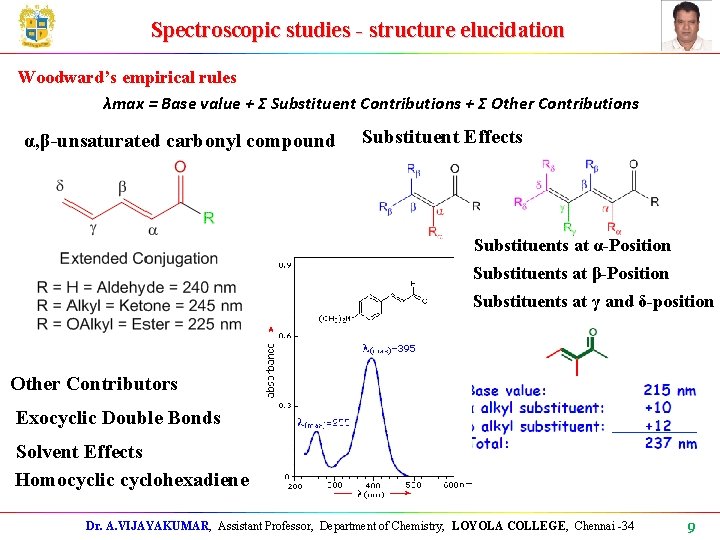
Spectroscopic studies - structure elucidation Woodward’s empirical rules λmax = Base value + Σ Substituent Contributions + Σ Other Contributions α, β-unsaturated carbonyl compound Substituent Effects Substituents at α-Position Substituents at β-Position Substituents at γ and δ-position Other Contributors Exocyclic Double Bonds Solvent Effects Homocyclic cyclohexadiene Dr. A. VIJAYAKUMAR, Assistant Professor, Department of Chemistry, LOYOLA COLLEGE, Chennai -34 9

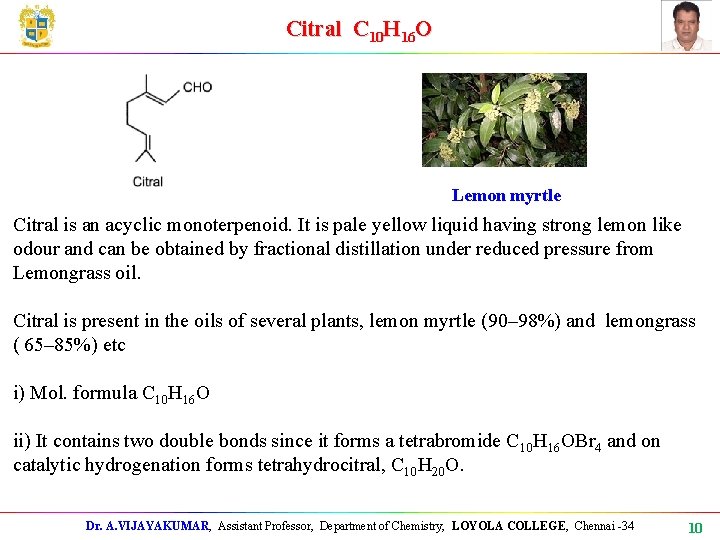
Citral C 10 H 16 O Lemon myrtle Citral is an acyclic monoterpenoid. It is pale yellow liquid having strong lemon like odour and can be obtained by fractional distillation under reduced pressure from Lemongrass oil. Citral is present in the oils of several plants, lemon myrtle (90– 98%) and lemongrass ( 65– 85%) etc i) Mol. formula C 10 H 16 O ii) It contains two double bonds since it forms a tetrabromide C 10 H 16 OBr 4 and on catalytic hydrogenation forms tetrahydrocitral, C 10 H 20 O. Dr. A. VIJAYAKUMAR, Assistant Professor, Department of Chemistry, LOYOLA COLLEGE, Chennai -34 10

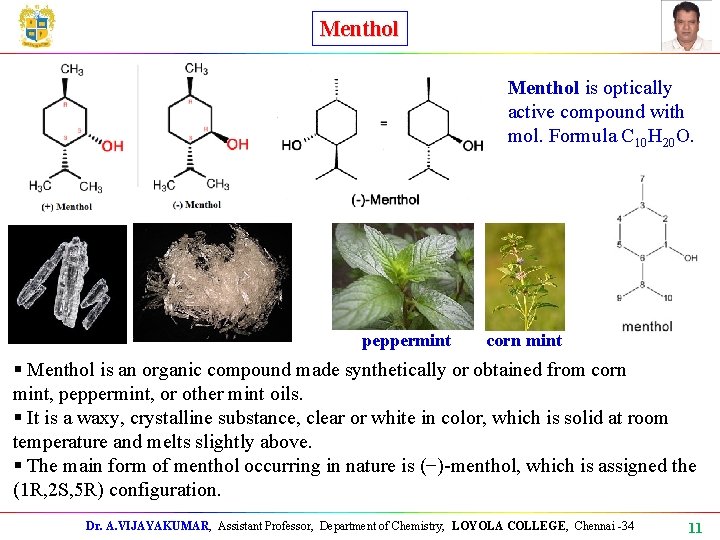
Menthol is optically active compound with mol. Formula C 10 H 20 O. peppermint corn mint § Menthol is an organic compound made synthetically or obtained from corn mint, peppermint, or other mint oils. § It is a waxy, crystalline substance, clear or white in color, which is solid at room temperature and melts slightly above. § The main form of menthol occurring in nature is (−)-menthol, which is assigned the (1 R, 2 S, 5 R) configuration. Dr. A. VIJAYAKUMAR, Assistant Professor, Department of Chemistry, LOYOLA COLLEGE, Chennai -34 11

Menthol - Biological properties Menthol's ability to chemically trigger the cold-sensitive receptors in the skin is responsible for the well-known cooling sensation it provokes when inhaled, eaten, or applied to the skin. Menthol also blocks voltage-sensitive sodium channels, reducing neural activity that may stimulate muscles. Menthol is widely used in dental care as a topical antibacterial agent. Menthol is included in many products for a variety of reasons Vicks Vapo. Rub ointment Mouthwash Toothpaste Chewing gum Lip balm Perfume Eucalyptus oil Tiger Balm Cough medicines Dr. A. VIJAYAKUMAR, Assistant Professor, Department of Chemistry, LOYOLA COLLEGE, Chennai -34 12

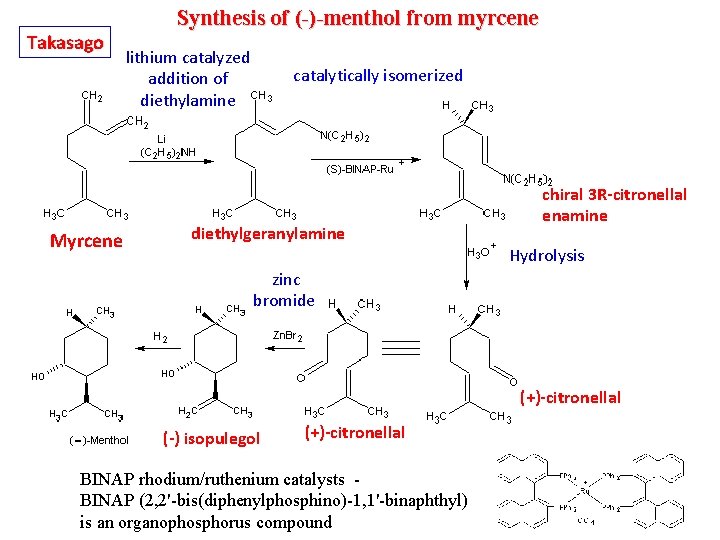
Takasago Synthesis of (-)-menthol from myrcene lithium catalyzed addition of diethylamine Myrcene catalytically isomerized diethylgeranylamine chiral 3 R-citronellal enamine Hydrolysis zinc bromide (+)-citronellal (-) isopulegol (+)-citronellal BINAP rhodium/ruthenium catalysts - BINAP (2, 2'-bis(diphenylphosphino)-1, 1'-binaphthyl) is an organophosphorus compound

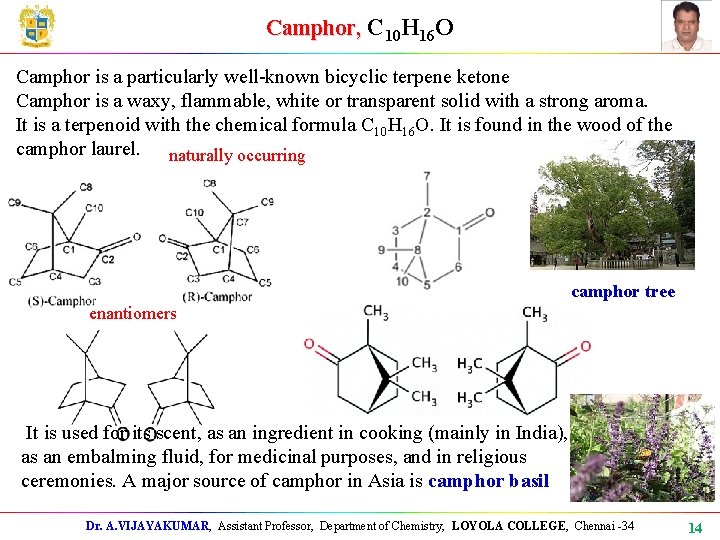
Camphor, C 10 H 16 O Camphor is a particularly well-known bicyclic terpene ketone Camphor is a waxy, flammable, white or transparent solid with a strong aroma. It is a terpenoid with the chemical formula C 10 H 16 O. It is found in the wood of the camphor laurel. naturally occurring camphor tree enantiomers It is used for its scent, as an ingredient in cooking (mainly in India), as an embalming fluid, for medicinal purposes, and in religious ceremonies. A major source of camphor in Asia is camphor basil Dr. A. VIJAYAKUMAR, Assistant Professor, Department of Chemistry, LOYOLA COLLEGE, Chennai -34 14

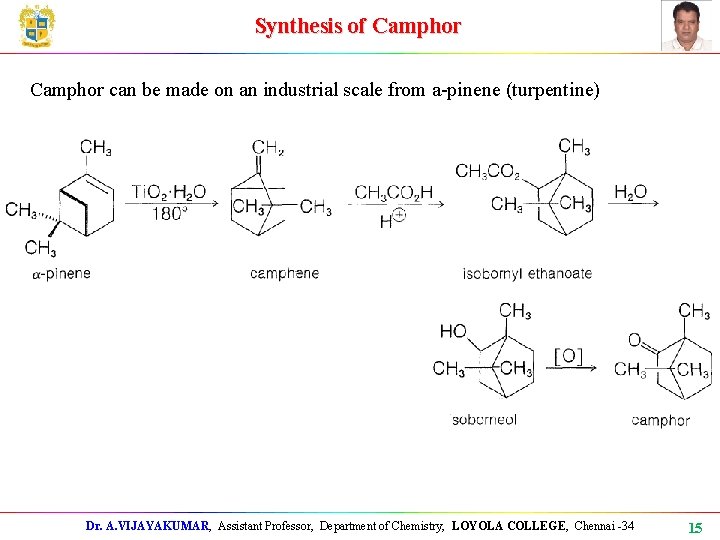
Synthesis of Camphor can be made on an industrial scale from a-pinene (turpentine) Dr. A. VIJAYAKUMAR, Assistant Professor, Department of Chemistry, LOYOLA COLLEGE, Chennai -34 15