TERAPIA CONVENZIONALE DEL LINFOMA FOLLICOLARE PIER LUIGI ZINZANI
















- Slides: 35

TERAPIA CONVENZIONALE DEL LINFOMA FOLLICOLARE PIER LUIGI ZINZANI Istituto di Ematologia e Oncologia “L. e A. Seràgnoli” Università degli Studi di Bologna Roma, 9 novembre 2006

SIAMO IN GRADO DI DEFINIRE LA MIGLIOR TERAPIA DI PRIMA LINEA NEL LINFOMA FOLLICOLARE?

Definire una strategia terapeutica nel linfoma follicolare Tener conto di: Obbiettivo Età Contenimento vs Cura < 60, 60 -75, > 75 Incremento RC Sopravvivenza più lunga? PCR negatività End point surrogato di una sopravvivenza più lunga?


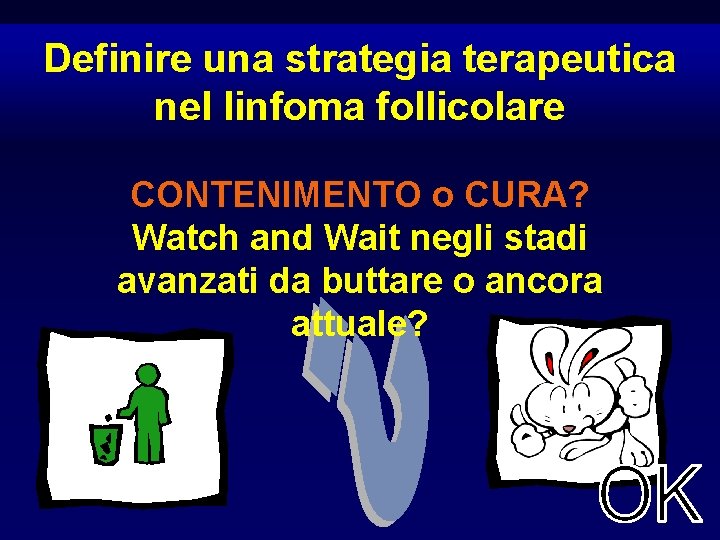
Definire una strategia terapeutica nel linfoma follicolare CONTENIMENTO o CURA? Watch and Wait negli stadi avanzati da buttare o ancora attuale?

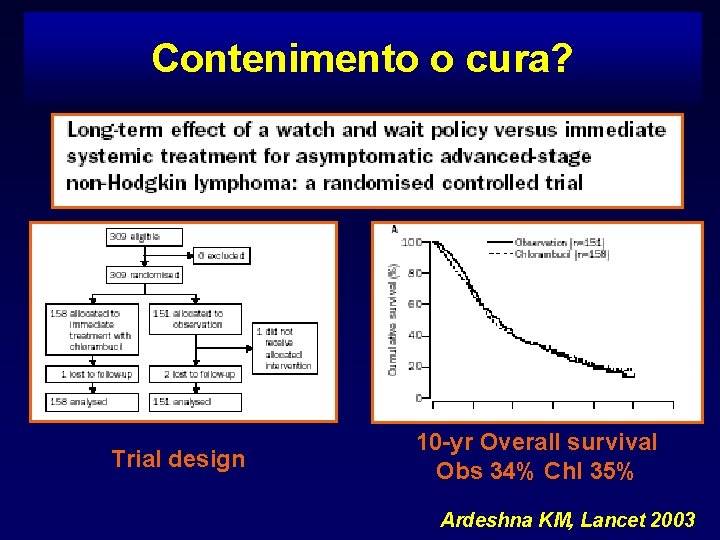
Contenimento o cura? Trial design 10 -yr Overall survival Obs 34% Chl 35% Ardeshna KM, Lancet 2003

Recommendation Treatment can be safely deferred without no disadvantage on survival for patients with stage III-IV disease, provided that none of the following features occurs: systemic symptoms, high tumor burden, extranodal disease, cytopenia due to marrow involvement, spleen involvement, leukemic phase, serous effusion, high LDH levels [grade A]. Linee Guida SIE, SIES, GITMO 2005

Panel’s considerations The results of the studies reported above suggest that a WW strategy may be still appropriate in selected cases with advanced disease since it allows to improve chemotherapy-free survival, especially in the elderly patients. Linee Guida SIE, SIES, GITMO 2005

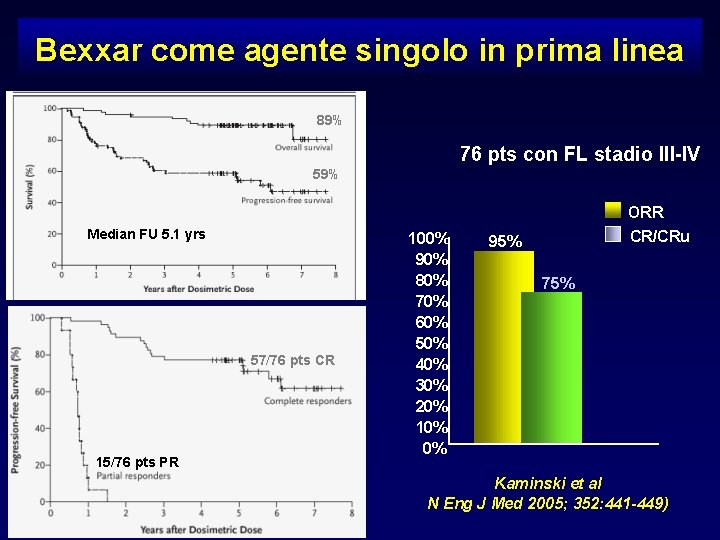
Bexxar come agente singolo in prima linea 89% 76 pts con FL stadio III-IV 59% ORR Median FU 5. 1 yrs 57/76 pts CR 15/76 pts PR 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% CR/CRu 95% 75% Kaminski et al N Eng J Med 2005; 352: 441 -449)

Linfoma follicolare COME TRATTARE ? Malattia localizzata Malattia avanzata

L. Follicolare: stadi iniziali Stadi I - II (<15 -20% dei casi) - basso tumor burden: radioterapia locoregionale (30 - 36 Gy) - alto tumor burden o rischio intermedio-alto (FLIPI>2) chemioterapia + radioterapia (front-line)

Linfoma Follicolare in stadio avanzato Terapie di prima linea Autore Terapia ORR RC Brandt 2001 Alchilante in monoterapia o CVP 60 – 70% 20 – 30% Dana 1993 CHOP-Like 60 – 70% 40 – 50% Tsimberidou 2002 FND vs ATT 83 - 94% 60 – 70% Foussard 2001 FM vs CHEP 86 vs 67% 34 vs 5% Zinzani 2002 FM vs CHOP 94 vs 93% 68 vs 37% OS 4. 5 – 9 anni 70 -80% a 5 aa

I Linfomi Follicolari in stadio avanzato sono curabili? Chemioterapia


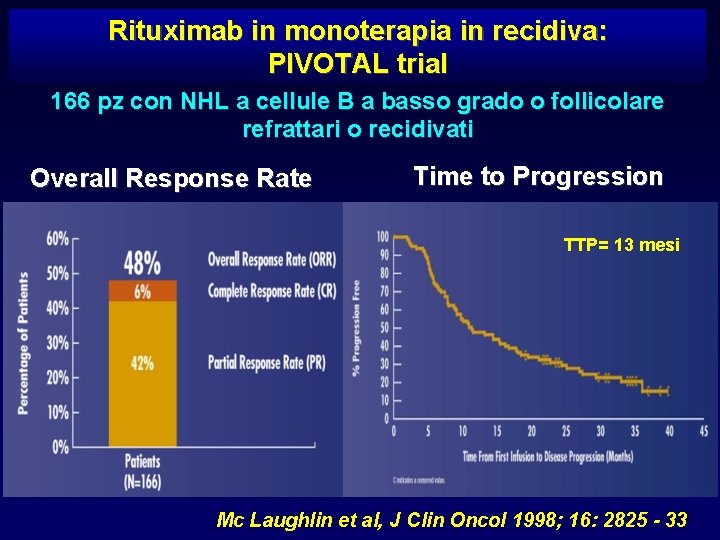
Rituximab in monoterapia in recidiva: PIVOTAL trial 166 pz con NHL a cellule B a basso grado o follicolare refrattari o recidivati Overall Response Rate Time to Progression TTP= 13 mesi Mc Laughlin et al, J Clin Oncol 1998; 16: 2825 - 33

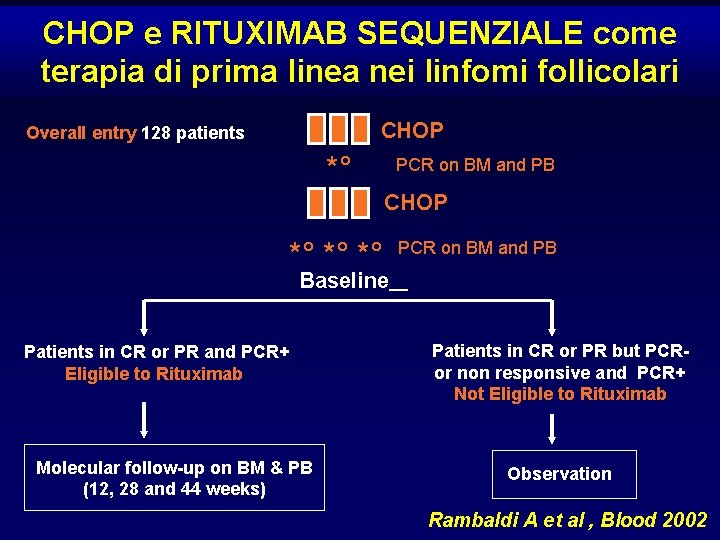
CHOP e RITUXIMAB SEQUENZIALE come terapia di prima linea nei linfomi follicolari CHOP Overall entry 128 patients *° PCR on BM and PB CHOP *° *° *° PCR on BM and PB Baseline Patients in CR or PR and PCR+ Eligible to Rituximab Molecular follow-up on BM & PB (12, 28 and 44 weeks) Patients in CR or PR but PCRor non responsive and PCR+ Not Eligible to Rituximab Observation Rambaldi A et al , Blood 2002

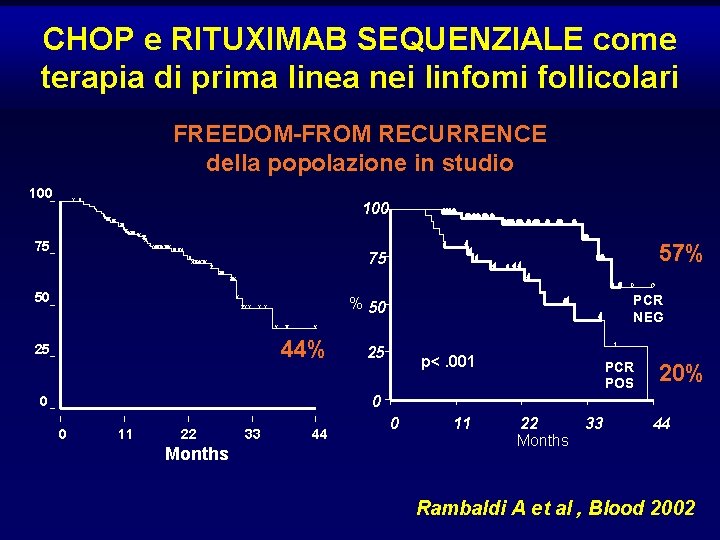
CHOP e RITUXIMAB SEQUENZIALE come terapia di prima linea nei linfomi follicolari FREEDOM-FROM RECURRENCE della popolazione in studio 100 75 57% 75 50 PCR NEG % 50 44% 25 25 p<. 001 PCR POS 20% 0 0 0 11 22 Months 33 44 0 11 22 33 Months 44 Rambaldi A et al , Blood 2002

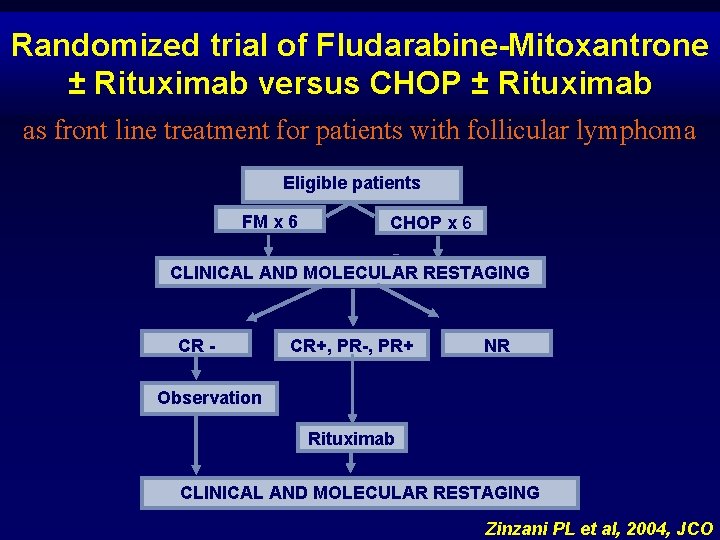
Randomized trial of Fludarabine-Mitoxantrone ± Rituximab versus CHOP ± Rituximab as front line treatment for patients with follicular lymphoma Eligible patients FM x 6 CHOP x 6 CLINICAL AND MOLECULAR RESTAGING CR - CR+, PR-, PR+ NR Observation Rituximab CLINICAL AND MOLECULAR RESTAGING Zinzani PL et al, 2004, JCO

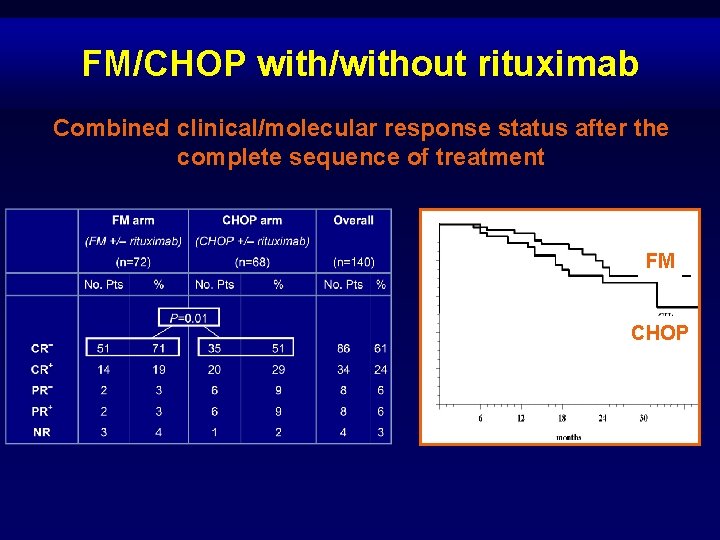
FM/CHOP with/without rituximab Combined clinical/molecular response status after the complete sequence of treatment FM CHOP

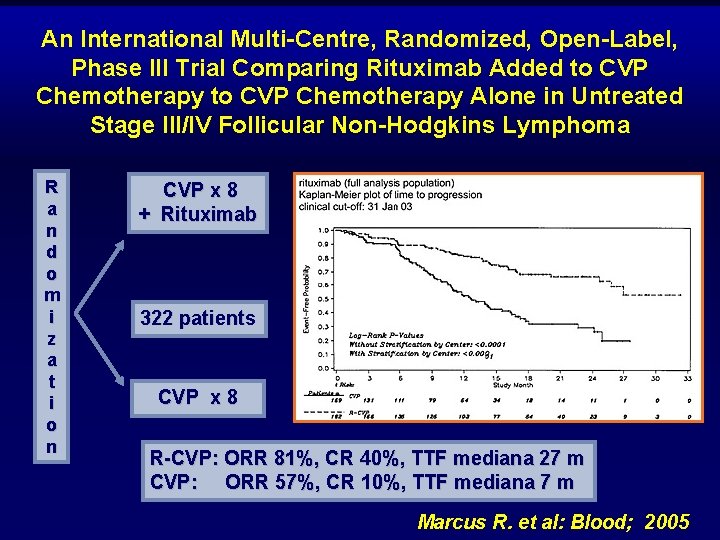
An International Multi-Centre, Randomized, Open-Label, Phase III Trial Comparing Rituximab Added to CVP Chemotherapy Alone in Untreated Stage III/IV Follicular Non-Hodgkins Lymphoma R a n d o m i z a t i o n CVP x 8 + Rituximab 322 patients CVP x 8 R-CVP: ORR 81%, CR 40%, TTF mediana 27 m CVP: ORR 57%, CR 10%, TTF mediana 7 m Marcus R. et al: Blood; 2005

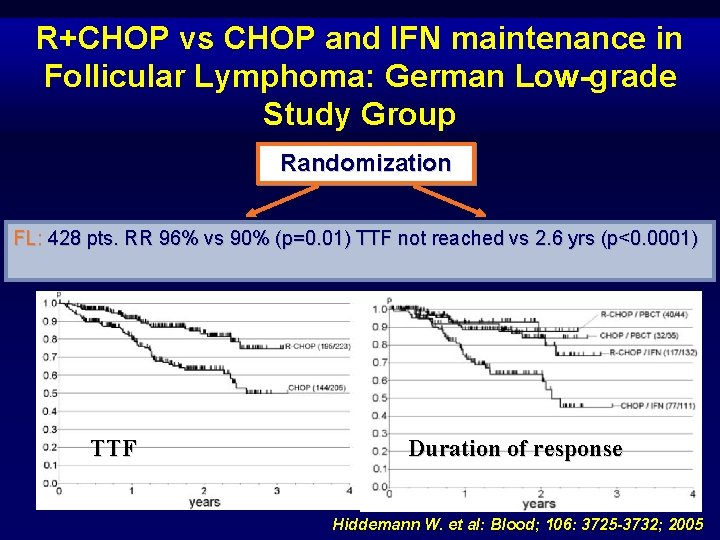
R+CHOP vs CHOP and IFN maintenance in Follicular Lymphoma: German Low-grade Study Group Randomization FL: 428 pts. RR 96%R-CHOP vs 90% (p=0. 01) TTF not reached vs 2. 6 yrs (p<0. 0001) CHOP Randomization IFN-a TTF maintenance Myeloablative RT-CT + PBCT pts <60 yr Duration of response Hiddemann W. et al: Blood; 106: 3725 -3732; 2005

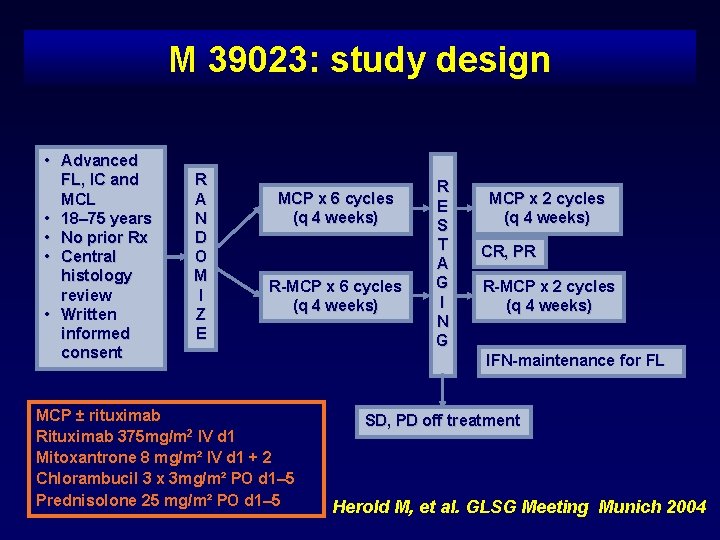
M 39023: study design • Advanced FL, IC and MCL • 18– 75 years • No prior Rx • Central histology review • Written informed consent R A N D O M I Z E MCP x 6 cycles (q 4 weeks) R-MCP x 6 cycles (q 4 weeks) MCP ± rituximab Rituximab 375 mg/m 2 IV d 1 Mitoxantrone 8 mg/m² IV d 1 + 2 Chlorambucil 3 x 3 mg/m² PO d 1– 5 Prednisolone 25 mg/m² PO d 1– 5 R E S T A G I N G MCP x 2 cycles (q 4 weeks) CR, PR R-MCP x 2 cycles (q 4 weeks) IFN-maintenance for FL SD, PD off treatment Herold M, et al. GLSG Meeting Munich 2004

M 39023: progression-free survival for FL patients (ITT population) Rituximab + MCP median not reached (88. 5% at 19. 7 months) Progression-free survival 1. 00 0. 75 MCP median 19. 7 months 0. 50 0. 25 p<0. 0001 0 0 10 20 Censored 30 Months 40 50 60 Herold M, et al. GLSG Meeting Munich 2004

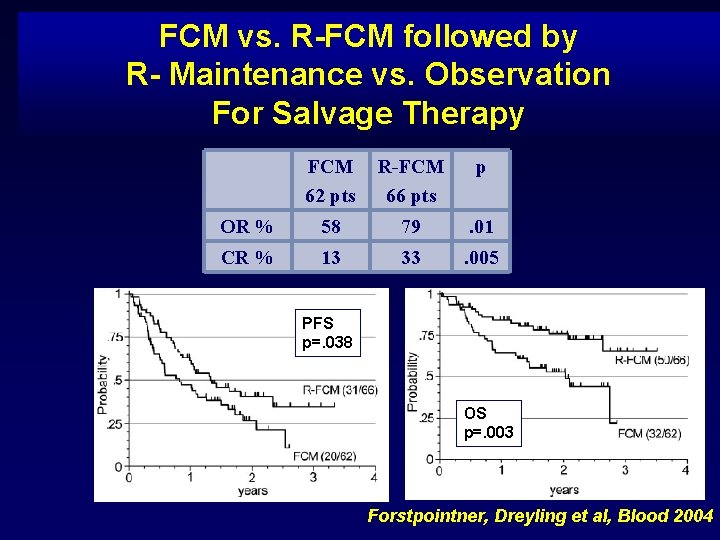
FCM vs. R-FCM followed by R- Maintenance vs. Observation For Salvage Therapy FCM 62 pts R-FCM 66 pts p OR % 58 79 . 01 CR % 13 33 . 005 PFS p=. 038 OS p=. 003 Forstpointner, Dreyling et al, Blood 2004

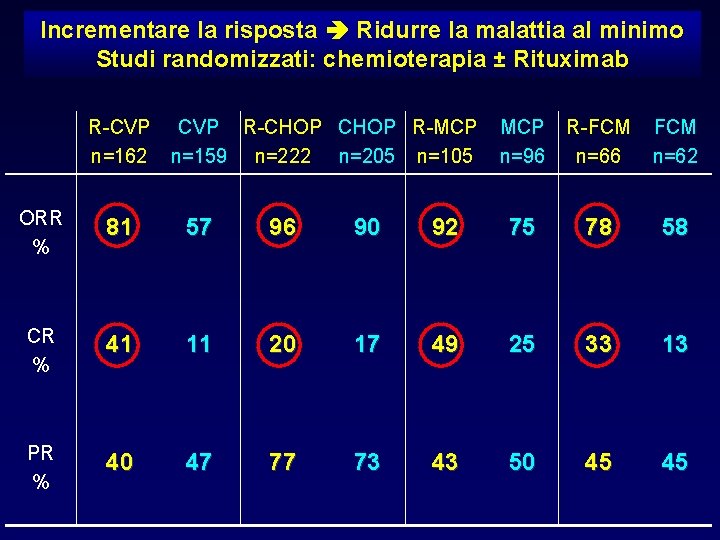
Incrementare la risposta Ridurre la malattia al minimo Studi randomizzati: chemioterapia ± Rituximab R-CVP n=162 CVP R-CHOP R-MCP n=159 n=222 n=205 n=105 MCP n=96 R-FCM n=66 FCM n=62 ORR % 81 57 96 90 92 75 78 58 CR % 41 11 20 17 49 25 33 13 PR % 40 47 77 73 43 50 45 45

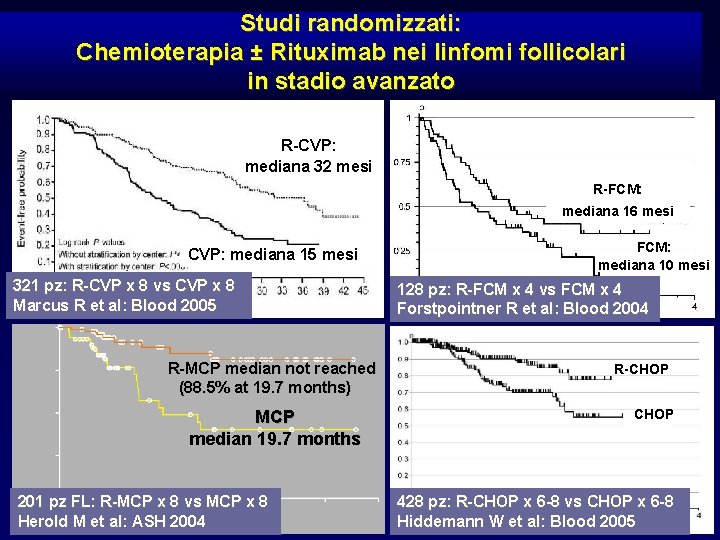
Studi randomizzati: Chemioterapia ± Rituximab nei linfomi follicolari in stadio avanzato R-CVP: mediana 32 mesi R-FCM: mediana 16 mesi FCM: mediana 10 mesi CVP: mediana 15 mesi 321 pz: R-CVP x 8 vs CVP x 8 Marcus R et al: Blood 2005 128 pz: R-FCM x 4 vs FCM x 4 Forstpointner R et al: Blood 2004 Progression-free survival 1. 00 0. 75 0. 50 0. 25 R-MCP median not reached (88. 5% at 19. 7 months) CHOP MCP median 19. 7 months 0 201 pz FL: R-MCP x 8 vs MCP x 8 0 20 40 Herold M et al: ASH 2004 50 R-CHOP 60 428 pz: R-CHOP x 6 -8 vs CHOP x 6 -8 Hiddemann W et al: Blood 2005

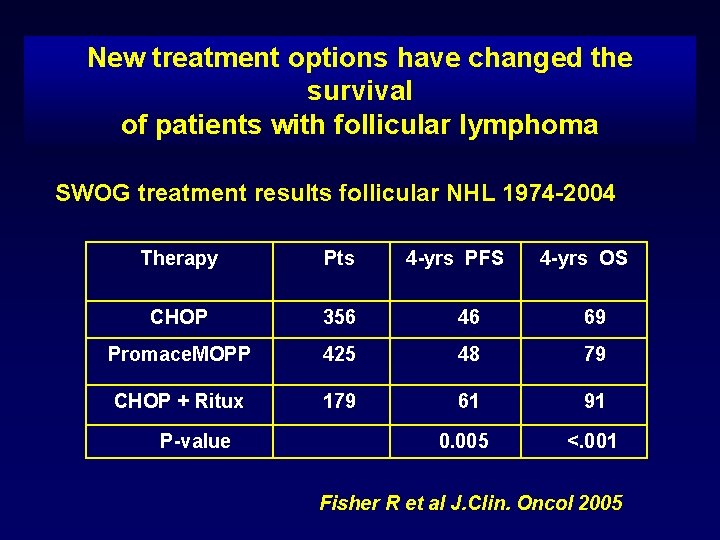
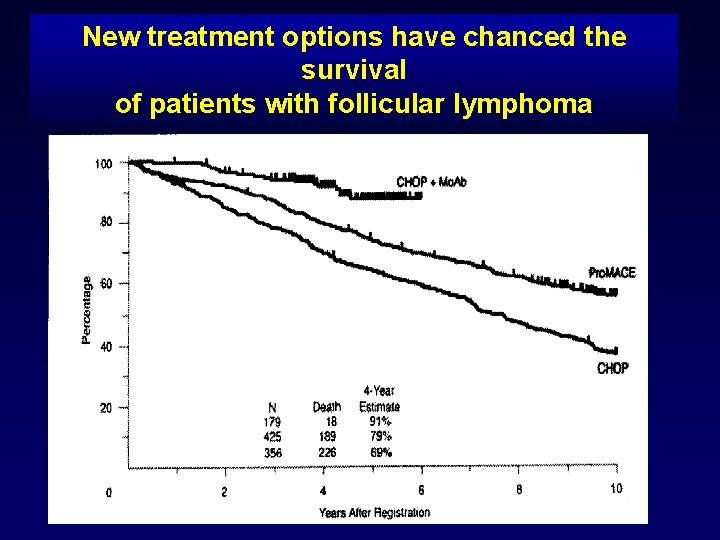
New treatment options have changed the survival of patients with follicular lymphoma SWOG treatment results follicular NHL 1974 -2004 Therapy Pts CHOP 356 46 69 Promace. MOPP 425 48 79 CHOP + Ritux 179 61 91 0. 005 <. 001 P-value 4 -yrs PFS 4 -yrs OS Fisher R et al J. Clin. Oncol 2005

New treatment options have chanced the survival of patients with follicular lymphoma

L. Follicolare in stadio avanzato: linee guida SIE L. follicolari 1 a linea • Frontline chemotherapy, either single-agent alkylators, antracyclines-based polychemotherapy or fludarabinebased polychemotherapy, should be chosen according to patient and disease characteristics [grade B]. • Rituximab concurrent or sequential, should be add to frontline conventional chemotherapy ( Grade B). • Younger patients should not receive single-agent alkylating chemotherapy because it is not able to induce molecular remission and reduce stem cell mobilization potential [grade C]. Haematologica, Sept. 2005

Terapia di 1 a linea del L. follicolare • L’immuno-chemioterapia è lo standard • Quale combinazione è più efficace? R-CVP, R-CHOP, R-FM • Necessari risultati di studi randomizzati R-CVP vs R-CHOP vs R-FM

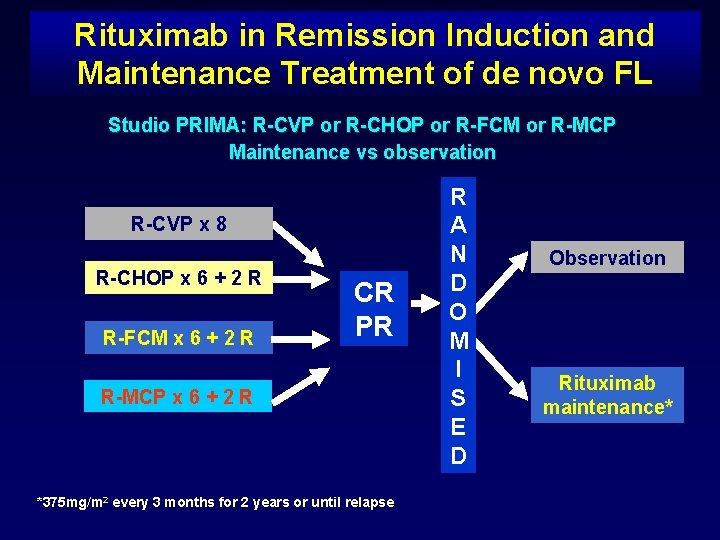
Rituximab in Remission Induction and Maintenance Treatment of de novo FL Studio PRIMA: R-CVP or R-CHOP or R-FCM or R-MCP Maintenance vs observation R-CVP x 8 R-CHOP x 6 + 2 R R-FCM x 6 + 2 R CR PR R-MCP x 6 + 2 R *375 mg/m 2 every 3 months for 2 years or until relapse R A N D O M I S E D Observation Rituximab maintenance*

Linfoma follicolare in stadio avanzato: problemi aperti § Significato e impatto prognostico della remissione molecolare § Terapie differenziate per rischio ed età? § Ruolo dell’auto-Tx in Ia remissione § Ruolo del mantenimento

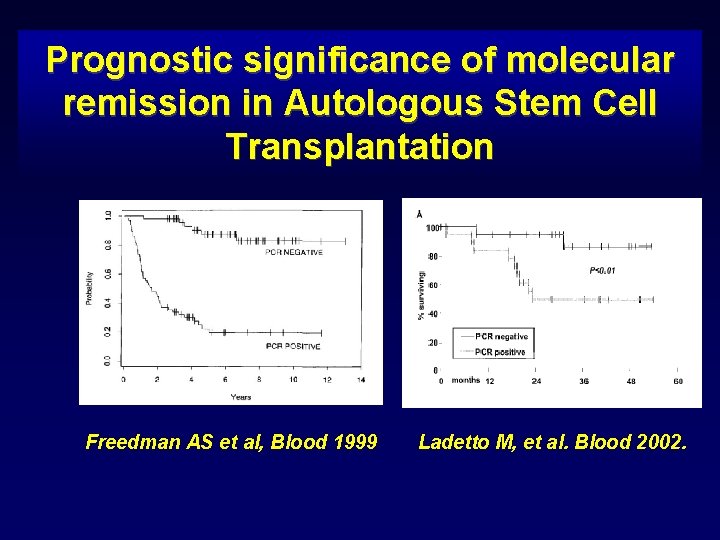
Prognostic significance of molecular remission in Autologous Stem Cell Transplantation Freedman AS et al, Blood 1999 Ladetto M, et al. Blood 2002.

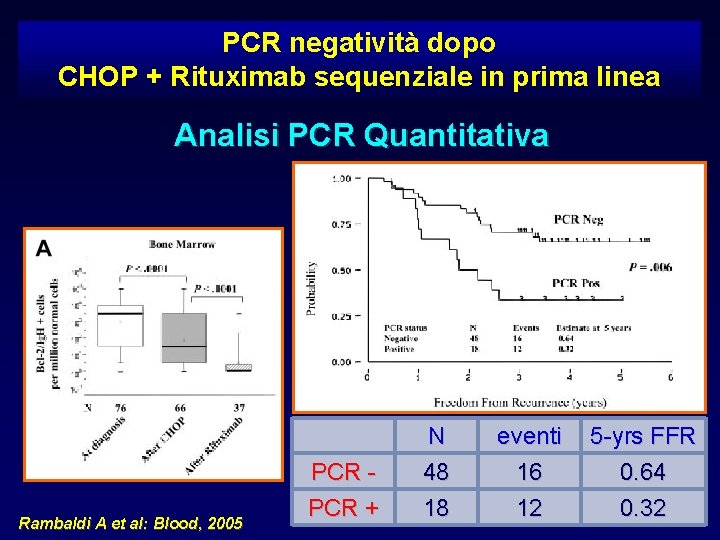
PCR negatività dopo CHOP + Rituximab sequenziale in prima linea Analisi PCR Quantitativa Rambaldi A et al: Blood, 2005 PCR + N 48 18 eventi 16 12 5 -yrs FFR 0. 64 0. 32

Linfoma follicolare in stadio avanzato: problemi aperti § Significato e impatto prognostico della remissione molecolare § Terapie differenziate per rischio ed età? § Ruolo dell’auto-Tx in Ia remissione § Ruolo del mantenimento
 Pier luigi zinzani
Pier luigi zinzani Pier luigi zinzani
Pier luigi zinzani Dott. pier luigi bruni
Dott. pier luigi bruni Pier luigi morara
Pier luigi morara ø matematica
ø matematica Circuito equivalente trasformatore
Circuito equivalente trasformatore Potenza dissipata formula
Potenza dissipata formula Elementi circuito elettrico
Elementi circuito elettrico Tomografia biomedicale
Tomografia biomedicale Sudorazioni notturne cause
Sudorazioni notturne cause Criterios de gelf
Criterios de gelf Linfoma folicular indolente
Linfoma folicular indolente Linfoma doble expresor
Linfoma doble expresor Linfoma de burkitt
Linfoma de burkitt Anaplasia tumorale
Anaplasia tumorale Linfoma marginale splenico
Linfoma marginale splenico Terapia educazionale del diabetico
Terapia educazionale del diabetico Deplaning curb
Deplaning curb A diver jumps up off a pier
A diver jumps up off a pier Pier pietro brunelli
Pier pietro brunelli Pierpass
Pierpass Pier
Pier I.c. pier delle vigne capua
I.c. pier delle vigne capua Pier delle vigne parafrasi
Pier delle vigne parafrasi Art
Art Dental bridge stress breaker
Dental bridge stress breaker Adjective clauses
Adjective clauses Fpd components
Fpd components Early christian design
Early christian design Pier lorenzo puri
Pier lorenzo puri Le arpie eneide parafrasi
Le arpie eneide parafrasi Eddies and ruby
Eddies and ruby Pier example
Pier example Pier giuseppe rossi
Pier giuseppe rossi Pier 23 navarre
Pier 23 navarre Pier giuseppe rossi
Pier giuseppe rossi