Tendyne Design Features and Clinical Update Neil Moat










- Slides: 29

Tendyne Design Features and Clinical Update Neil Moat Royal Brompton Hospital, London, UK

Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship Consulting Fees/Honoraria Company • Medtronic, Abbott, Direct Flow Medical, Edwards

Tendyne Transcatheter Mitral Valve Self-Expanding Nitinol Double Frame • Symmetrical Tri-Leaflet Porcine Pericardial Valve • Large Valve Size Matrix • Single inner valve size • Multiple outer frame sizes • Large Effective Orifice Area • Valve Tether to Apex • Adjustable tension provides valve stability • Apical Pad assists in access closure

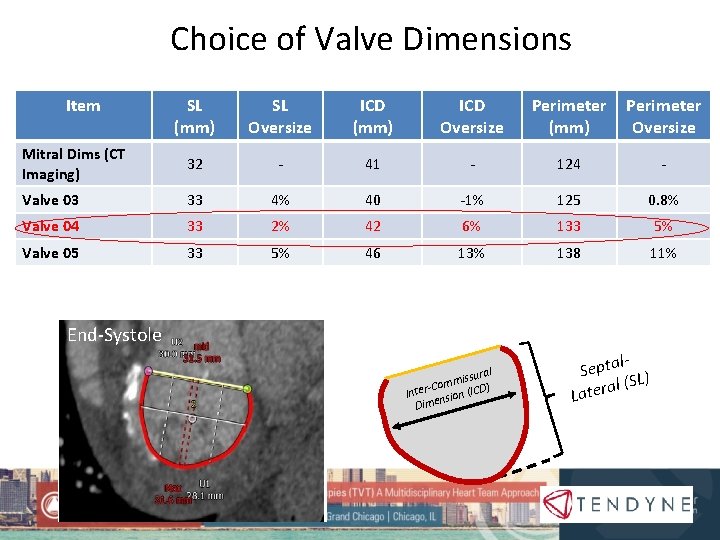
Choice of Valve Dimensions Item SL (mm) SL Oversize ICD (mm) ICD Oversize Perimeter (mm) Perimeter Oversize Mitral Dims (CT Imaging) 32 - 41 - 124 - Valve 03 33 4% 40 -1% 125 0. 8% Valve 04 33 2% 42 6% 133 5% Valve 05 33 5% 46 13% 138 11% End-Systole l issura m m Co ) Inter- nsion (ICD Dime l. Septa L) l (S a r e t La




30 Mar 2016 Tendyne Proprietary and Confidential 8

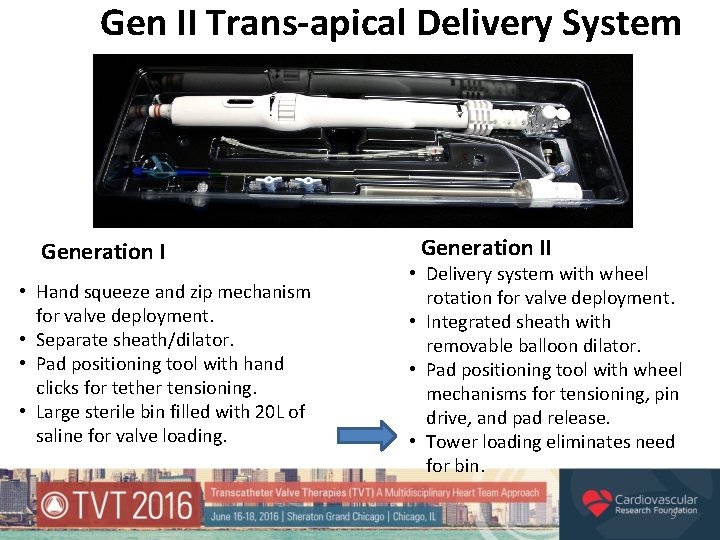
Gen II Trans-apical Delivery System Generation I • Hand squeeze and zip mechanism for valve deployment. • Separate sheath/dilator. • Pad positioning tool with hand clicks for tether tensioning. • Large sterile bin filled with 20 L of saline for valve loading. Generation II • Delivery system with wheel rotation for valve deployment. • Integrated sheath with removable balloon dilator. • Pad positioning tool with wheel mechanisms for tensioning, pin drive, and pad release. • Tower loading eliminates need for bin. 9


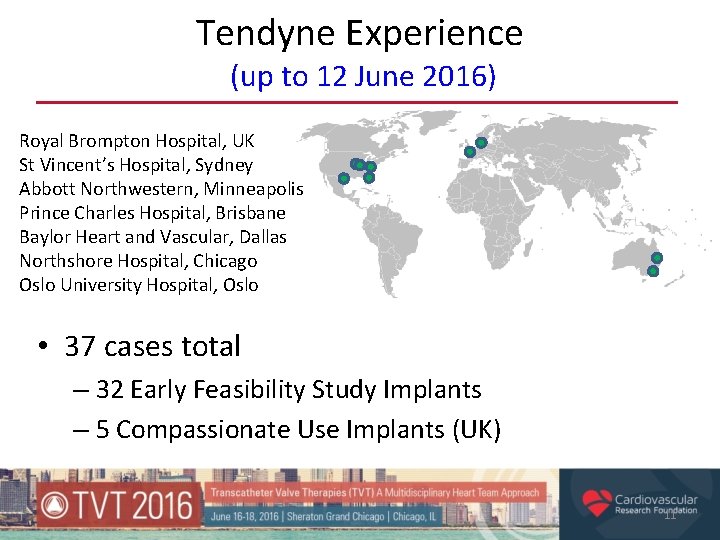
Tendyne Experience (up to 12 June 2016) Royal Brompton Hospital, UK St Vincent’s Hospital, Sydney Abbott Northwestern, Minneapolis Prince Charles Hospital, Brisbane Baylor Heart and Vascular, Dallas Northshore Hospital, Chicago Oslo University Hospital, Oslo • 37 cases total – 32 Early Feasibility Study Implants – 5 Compassionate Use Implants (UK) 11

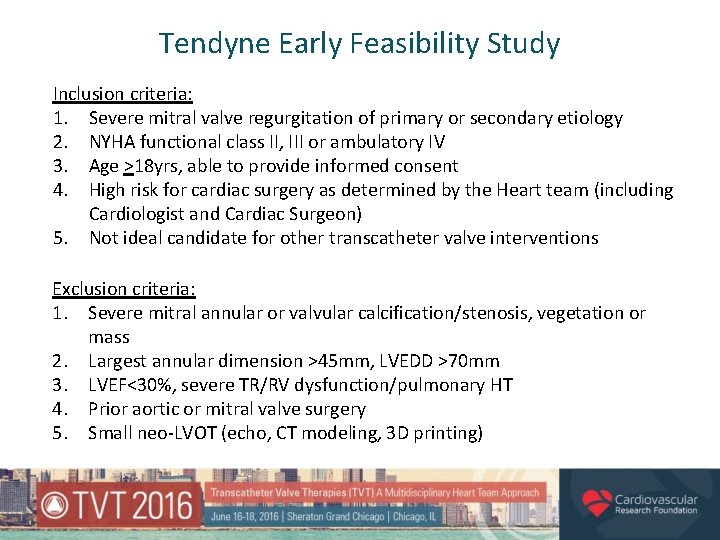
Tendyne Early Feasibility Study Inclusion criteria: 1. Severe mitral valve regurgitation of primary or secondary etiology 2. NYHA functional class II, III or ambulatory IV 3. Age >18 yrs, able to provide informed consent 4. High risk for cardiac surgery as determined by the Heart team (including Cardiologist and Cardiac Surgeon) 5. Not ideal candidate for other transcatheter valve interventions Exclusion criteria: 1. Severe mitral annular or valvular calcification/stenosis, vegetation or mass 2. Largest annular dimension >45 mm, LVEDD >70 mm 3. LVEF<30%, severe TR/RV dysfunction/pulmonary HT 4. Prior aortic or mitral valve surgery 5. Small neo-LVOT (echo, CT modeling, 3 D printing)

Tendyne EFS: Demographics (n=23) (courtesy of D Muller) N=23 Age (Mean+SD) 76. 9+9. 0 years Age Range 55. 1 -91. 4 years Gender Male 21 (91. 3%) Female 2 (8. 7%) NYHA Functional Class II 11 (47. 8%) III 12 (52. 2%) IV 0 (0%) STS Score (Mean+SD) 9. 5+12. 8 (2. 0 -61. 0 )

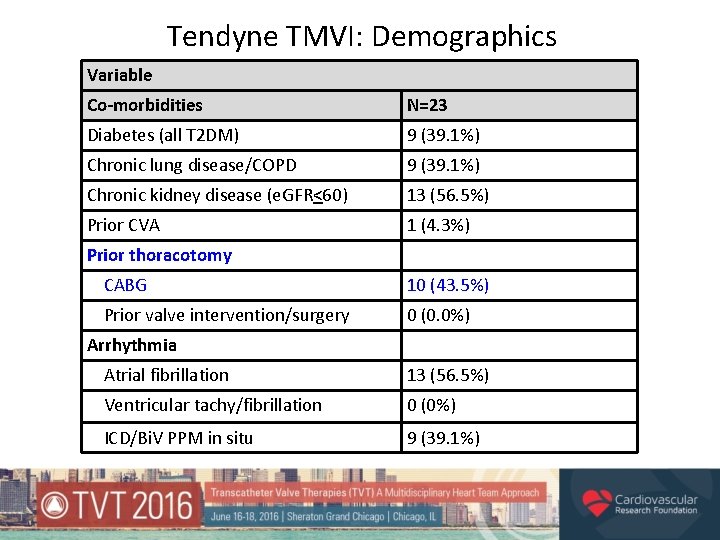
Tendyne TMVI: Demographics Variable Co-morbidities N=23 Diabetes (all T 2 DM) 9 (39. 1%) Chronic lung disease/COPD 9 (39. 1%) Chronic kidney disease (e. GFR<60) 13 (56. 5%) Prior CVA 1 (4. 3%) Prior thoracotomy CABG 10 (43. 5%) Prior valve intervention/surgery 0 (0. 0%) Arrhythmia Atrial fibrillation 13 (56. 5%) Ventricular tachy/fibrillation 0 (0%) ICD/Bi. V PPM in situ 9 (39. 1%)

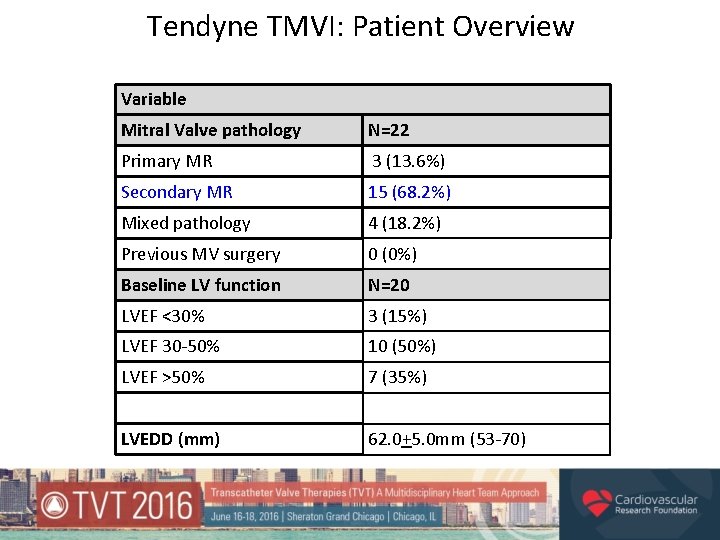
Tendyne TMVI: Patient Overview Variable Mitral Valve pathology N=22 Primary MR 3 (13. 6%) Secondary MR 15 (68. 2%) Mixed pathology 4 (18. 2%) Previous MV surgery 0 (0%) Baseline LV function N=20 LVEF <30% 3 (15%) LVEF 30 -50% 10 (50%) LVEF >50% 7 (35%) LVEDD (mm) 62. 0+5. 0 mm (53 -70)

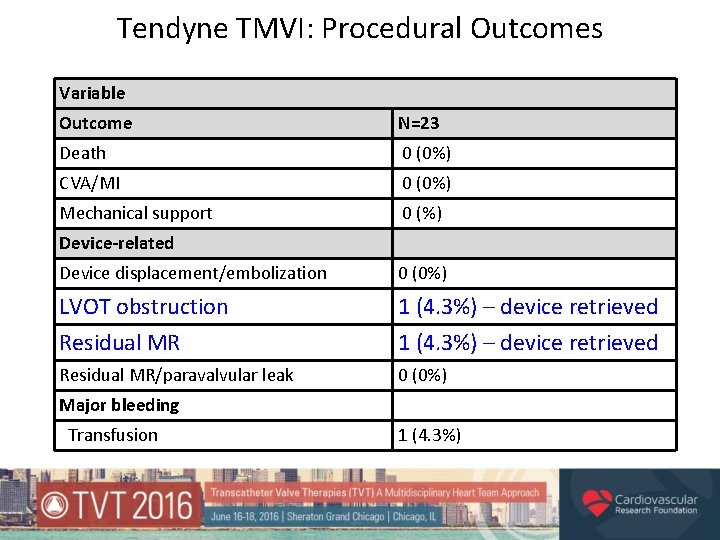
Tendyne TMVI: Procedural Outcomes Variable Outcome N=23 Death 0 (0%) CVA/MI 0 (0%) Mechanical support 0 (%) Device-related Device displacement/embolization 0 (0%) LVOT obstruction Residual MR 1 (4. 3%) – device retrieved Residual MR/paravalvular leak 0 (0%) Major bleeding Transfusion 1 (4. 3%)

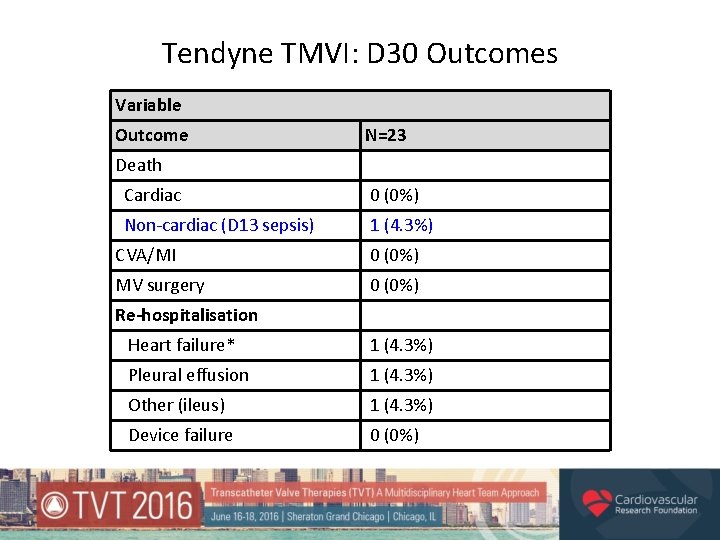
Tendyne TMVI: D 30 Outcomes Variable Outcome N=23 Death Cardiac 0 (0%) Non-cardiac (D 13 sepsis) 1 (4. 3%) CVA/MI 0 (0%) MV surgery 0 (0%) Re-hospitalisation Heart failure* 1 (4. 3%) Pleural effusion 1 (4. 3%) Other (ileus) 1 (4. 3%) Device failure 0 (0%)

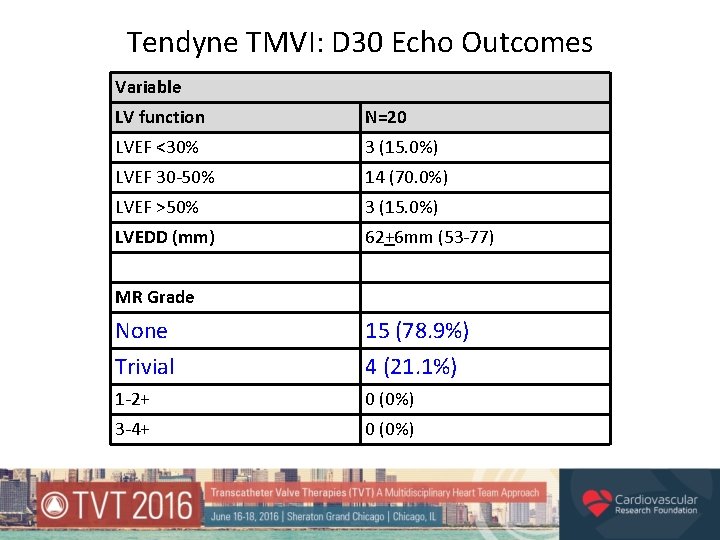
Tendyne TMVI: D 30 Echo Outcomes Variable LV function N=20 LVEF <30% 3 (15. 0%) LVEF 30 -50% 14 (70. 0%) LVEF >50% 3 (15. 0%) LVEDD (mm) 62+6 mm (53 -77) MR Grade None Trivial 15 (78. 9%) 4 (21. 1%) 1 -2+ 0 (0%) 3 -4+ 0 (0%)

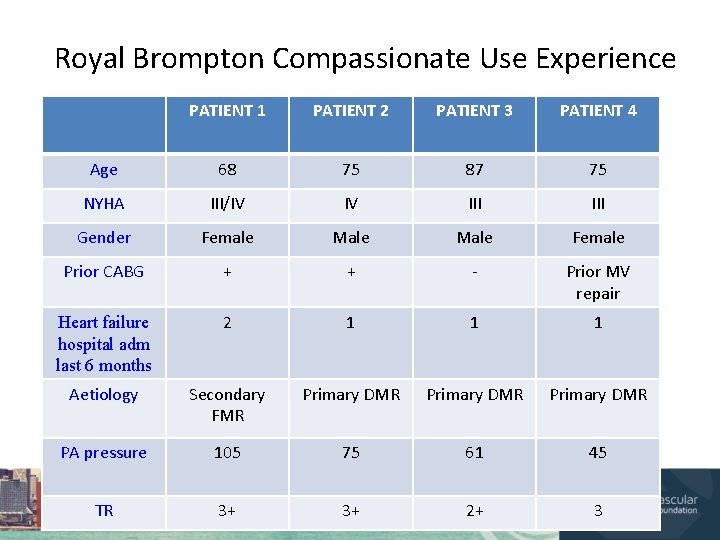
Royal Brompton Compassionate Use Experience PATIENT 1 PATIENT 2 PATIENT 3 PATIENT 4 Age 68 75 87 75 NYHA III/IV IV III Gender Female Male Female Prior CABG + + - Prior MV repair Heart failure hospital adm last 6 months 2 1 1 1 Aetiology Secondary FMR Primary DMR PA pressure 105 75 61 45 TR 3+ 3+ 2+ 3


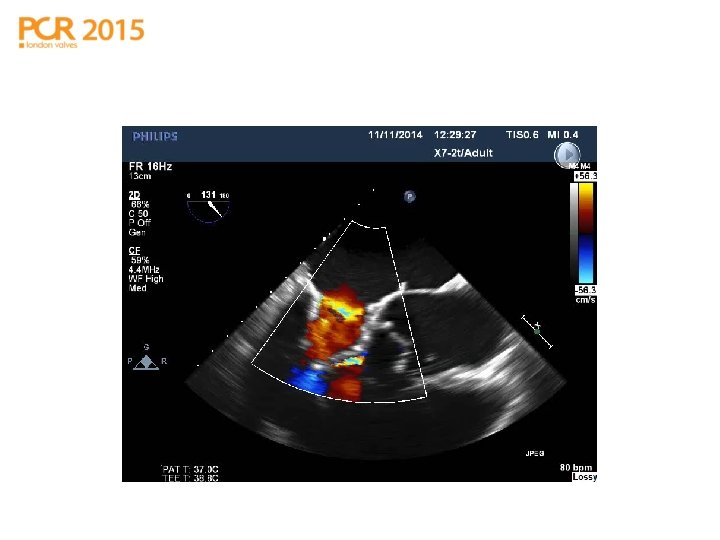
TMVI in place: LVOTO & SAM

TMVI in place: SAM


LVOT stenting

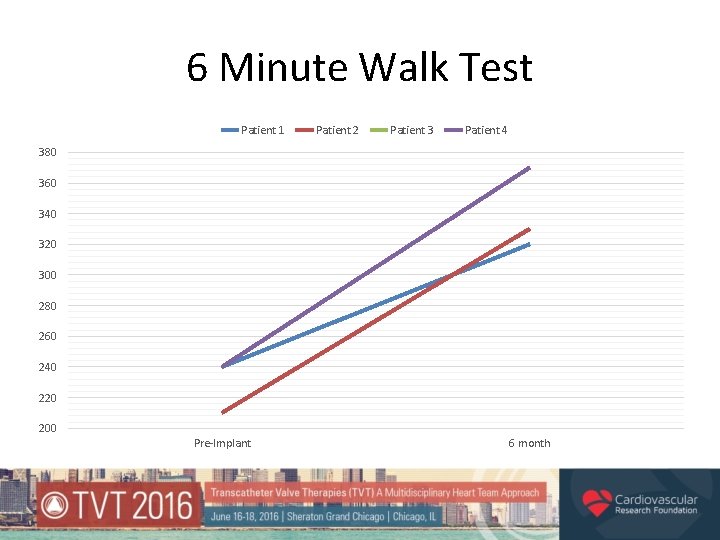
6 Minute Walk Test Patient 1 Patient 2 Patient 3 Patient 4 380 360 340 320 300 280 260 240 220 200 Pre-Implant 6 month

Tricuspid Regurgitation 4 3. 5 3 2. 5 TR GRADE 2 1. 5 1 0. 5 0 Pre-Implant Patient 1 6 Month Patient 2 Patient 3 Patient 4

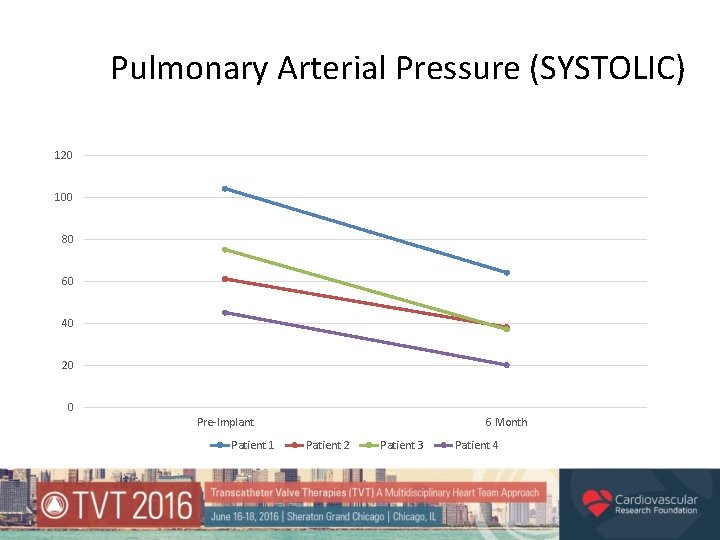
Pulmonary Arterial Pressure (SYSTOLIC) 120 100 80 60 40 20 0 Pre-Implant Patient 1 6 Month Patient 2 Patient 3 Patient 4

Royal Brompton Compassionate Use Experience • At 6 months (N=5) – all showed improved 6 MWT and a reduction in TR and PAP • At 18 months (N=3) – sustained improvement, no device migration, instability or dysfunction • No late development of new PVL • 1 death at 9 months

Tendyne TMVI - Summary • Fully retrievable, repositionable device • Predictable, controlled deployment • Well tolerated haemodynamically • Effective at reducing MR to nil in vast majority of patients • Apical pad provides stability and aids access site closure • Valve is stable post-deployment (out to 18 months in CU study) • No evidence of late (new) paravalvar leaks • Expanded Global Trial currently enrolling