Temperature vs Heat vs Internal Energy 1 2









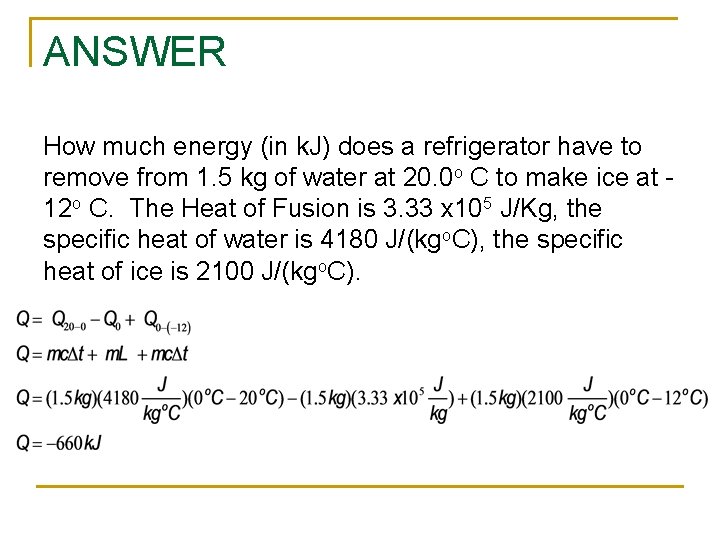

- Slides: 41

Temperature vs. Heat vs. Internal Energy 1. 2. 3. 4. Work in groups in 2 -3 Collect a whiteboard and pens Make a Venn Diagram showing how the terms Temperature, Heat and Internal Energy are similar and different Use your notes and phones for resources!

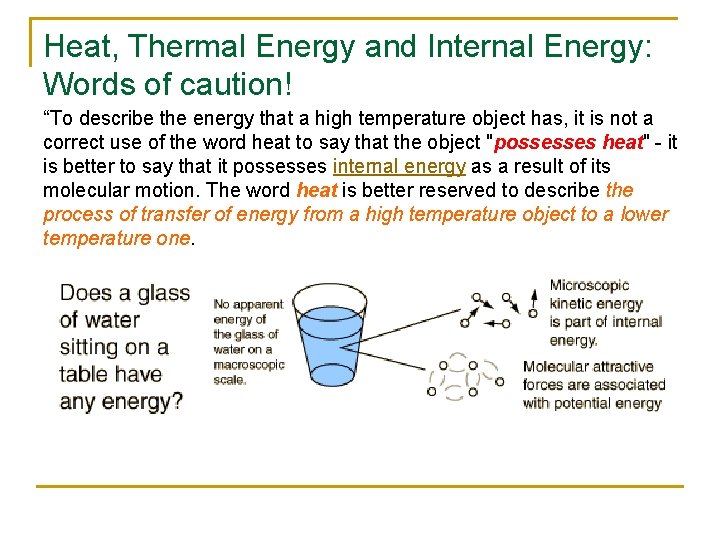
Heat, Thermal Energy and Internal Energy: Words of caution! “To describe the energy that a high temperature object has, it is not a correct use of the word heat to say that the object "possesses heat" - it is better to say that it possesses internal energy as a result of its molecular motion. The word heat is better reserved to describe the process of transfer of energy from a high temperature object to a lower temperature one.

Thermal Physics AP Physics B

Thermal Physics n Temperature and Heat q Mechanical equivalent of heat n n q Heat transfer and thermal expansion n n Zeroth Law of Thermodynamics Latent Heat Conduction Convection Radiation Kinetic Theory and Thermodynamics q Ideal gases n n q Kinetic model Ideal gas law Laws of thermodynamics n n First law (including processes on p. V diagrams) Second law (including heat engines)

Temperature and Heat Temperature: physical property of matter that quantitatively expresses the common notions of hot and cold. q The temperature varies with the microscopic speed of the fundamental particles that it contains (or their kinetic energy).

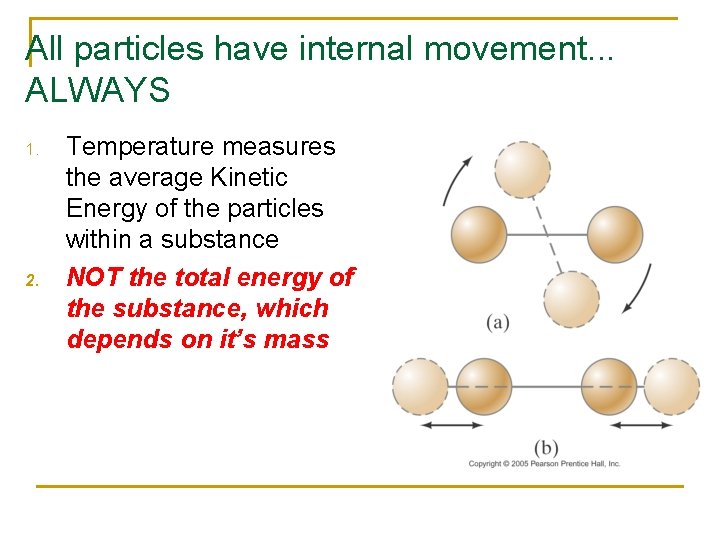
All particles have internal movement. . . ALWAYS 1. 2. Temperature measures the average Kinetic Energy of the particles within a substance NOT the total energy of the substance, which depends on it’s mass

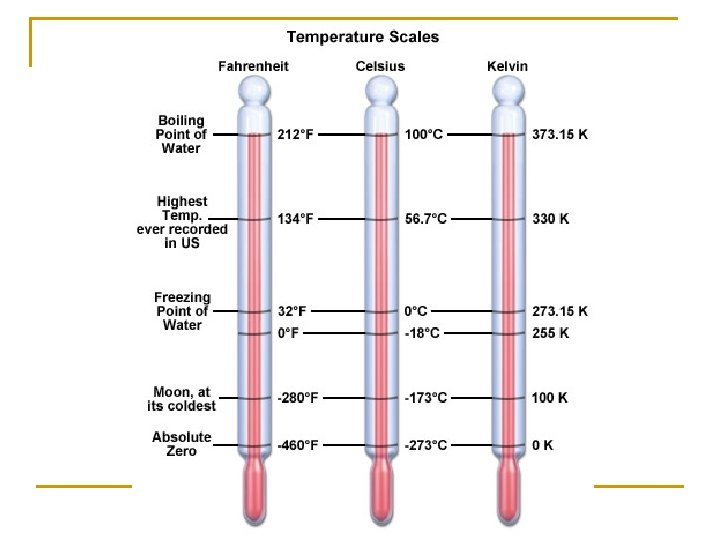
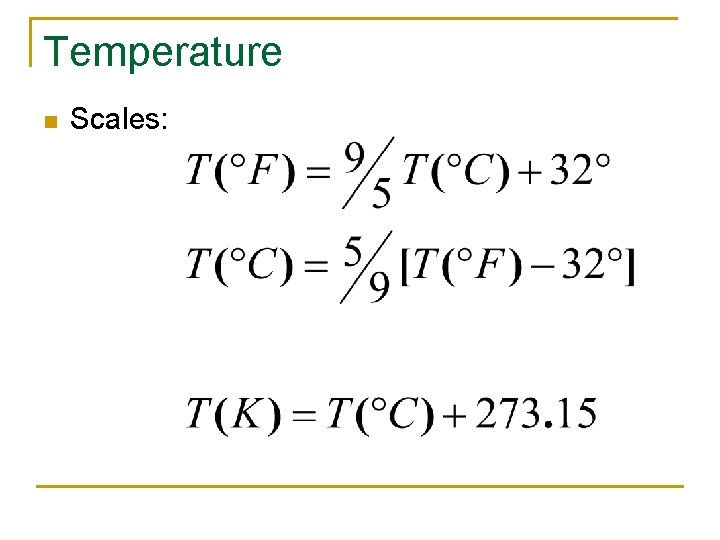
Basis for Temp Scales n n n Fahrenheit: Oldest scale, Freezing point is 32 o. F, Boiling point 212 o. F ~ makes no sense… Celsius: Water Freezes/Melts at 0 o. C, and boils at 100 o. C Kelvin: 0 o. K is the coolest theoretical temperature possible, no negative Kelvins. Same increments at Celsius Scale. Basically an updated version of the Celsius scale


Temperature n Scales:

Absolute Zero – 0 Kelvin Q: What would have to happen in order to reach absolute zero? n Atoms and subatomic particles would have to stop moving… impossible. n 2003 - MIT scientists cooled sodium gas to the lowest temperature ever recorded -- only half-a-billionth of a degree above absolute zero.

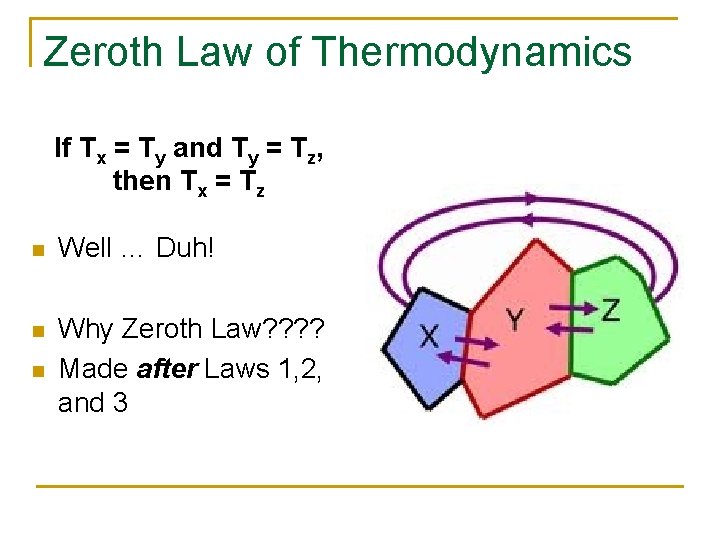
Zeroth Law of Thermodynamics If Tx = Ty and Ty = Tz, then Tx = Tz n Well … Duh! n Why Zeroth Law? ? Made after Laws 1, 2, and 3 n

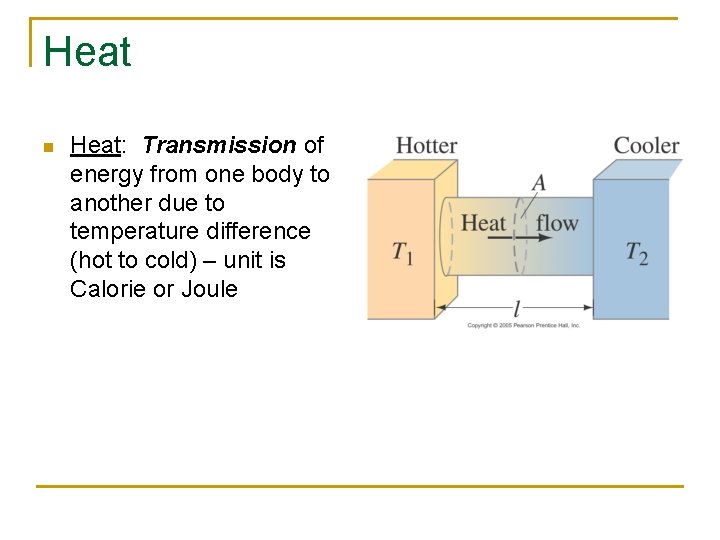
Heat n Heat: Transmission of energy from one body to another due to temperature difference (hot to cold) – unit is Calorie or Joule

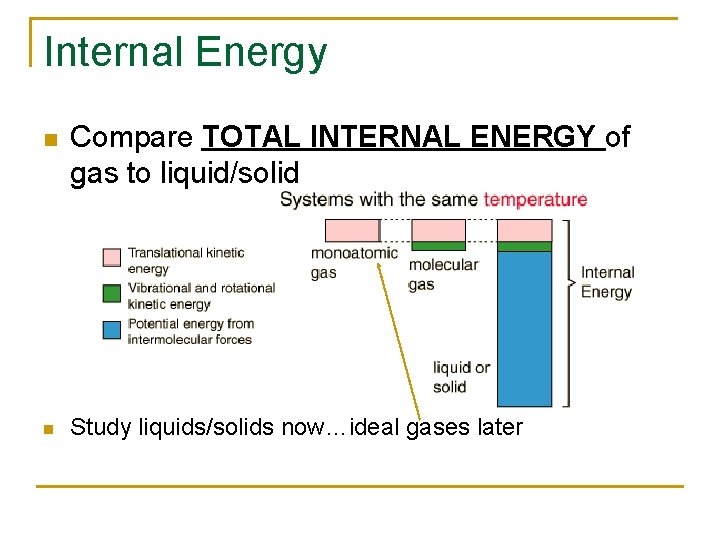
Internal Energy n n Compare TOTAL INTERNAL ENERGY of gas to liquid/solid Study liquids/solids now…ideal gases later

Heat n Take Home Message(s) q Heat is a process n n Matter contains internal energy NOT HEAT Heat is the transfer or conversion of energy q Compare to Work and Mechanical Energy…

In-Class Work + Homework n Collect the worksheet from the front of the room If you haven’t already… please watch the Video – 2 for homework! + MC Q’s: 6, 24, 30, 32, 35, 39, 58 n ALSO: Watch Specific Heat Video if you need a review from Phys 11! n

Heat Transfer Due to ΔT n All materials are not created equally in terms of heat transfer q q Would you rather touch your tongue to a 0 o. C metal pole or wooden pole? Why? (Splinters? )

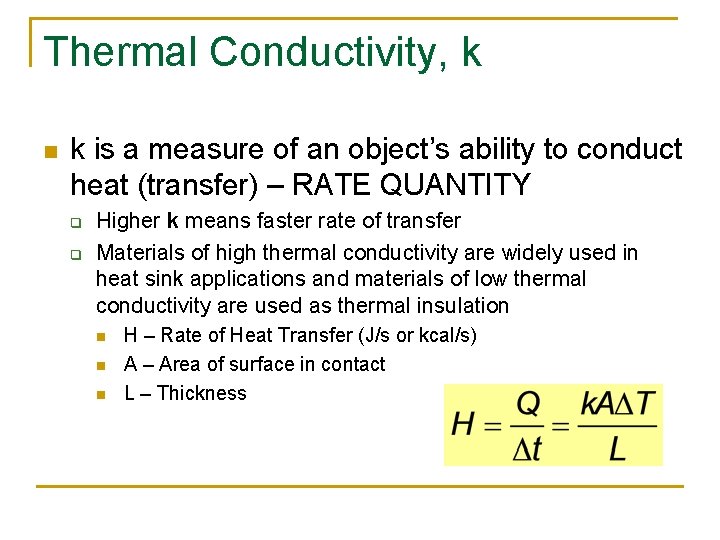
Thermal Conductivity, k n k is a measure of an object’s ability to conduct heat (transfer) – RATE QUANTITY q q Higher k means faster rate of transfer Materials of high thermal conductivity are widely used in heat sink applications and materials of low thermal conductivity are used as thermal insulation n H – Rate of Heat Transfer (J/s or kcal/s) A – Area of surface in contact L – Thickness

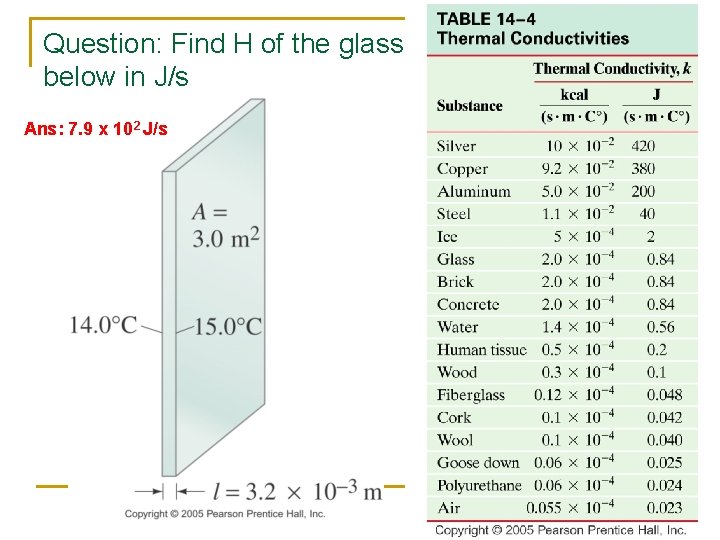
Question: Find H of the glass below in J/s Ans: 7. 9 x 102 J/s

Question What is a better insulator, an object with a larger k, or smaller k?

Question What is a better insulator, an object with a larger k, or smaller k? n Ans: Smaller k! Some questions are not difficult

Question If air has such a low thermal conductivity (0. 22), why do we need to wear clothes? (Other than for decency reasons…)

Question If air has such a low thermal conductivity (0. 22), why do we need to wear clothes? (Other than for decency reasons…) ANS: Air is always moving, we use clothes to trap air close to our bodies. The thicker the clothes the more air we trap.

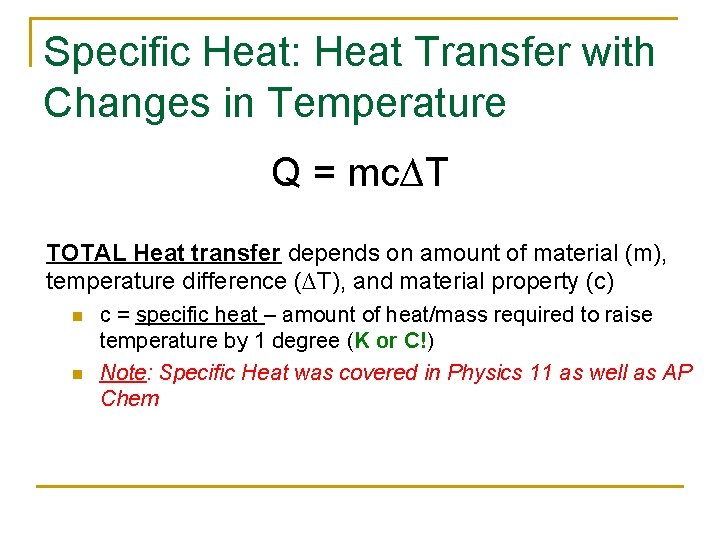
Specific Heat: Heat Transfer with Changes in Temperature Q = mc∆T TOTAL Heat transfer depends on amount of material (m), temperature difference (∆T), and material property (c) n n c = specific heat – amount of heat/mass required to raise temperature by 1 degree (K or C!) Note: Specific Heat was covered in Physics 11 as well as AP Chem

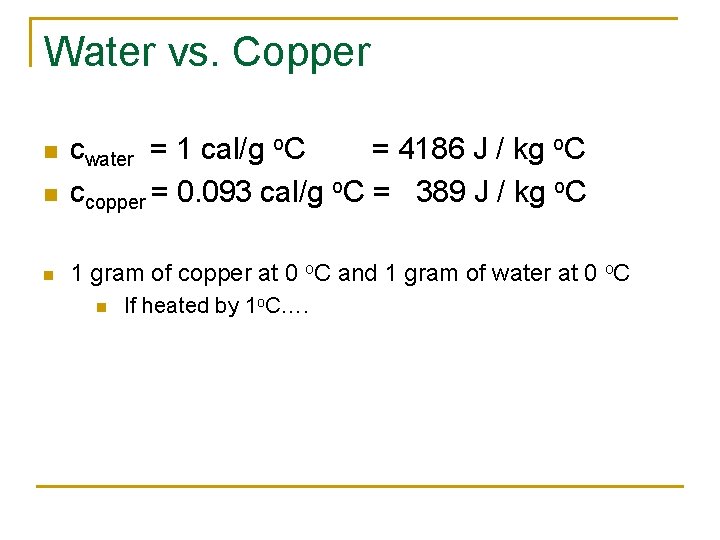
Water vs. Copper n cwater = 1 cal/g o. C = 4186 J / kg o. C ccopper = 0. 093 cal/g o. C = 389 J / kg o. C n 1 gram of copper at 0 o. C and 1 gram of water at 0 o. C n n If heated by 1 o. C….

Specific Heat: Heat Transfer with Changes in Temp ∆T= Q/mc n Same Q transfers, different objects (ignore -/+!) q Big mass vs. small mass: small mass will get hotter q Metal (lower c) vs. Wood (higher c): metal gets hotter

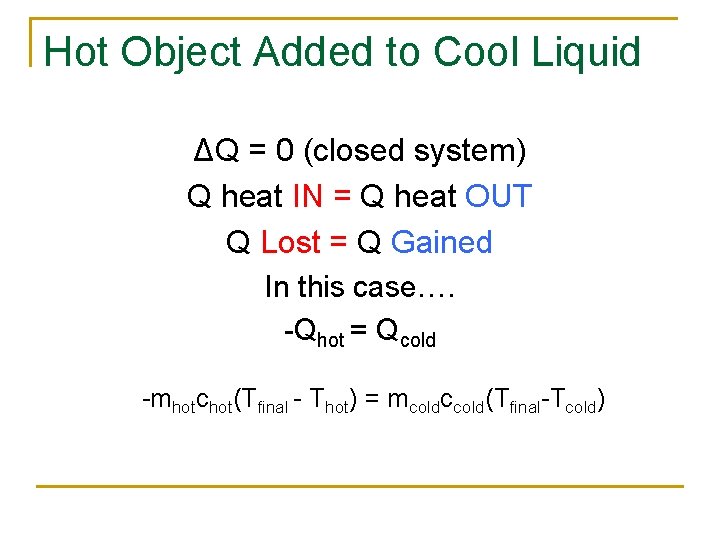
Hot Object Added to Cool Liquid ΔQ = 0 (closed system) Q heat IN = Q heat OUT Q Lost = Q Gained In this case…. -Qhot = Qcold -mhotchot(Tfinal - Thot) = mcoldccold(Tfinal-Tcold)

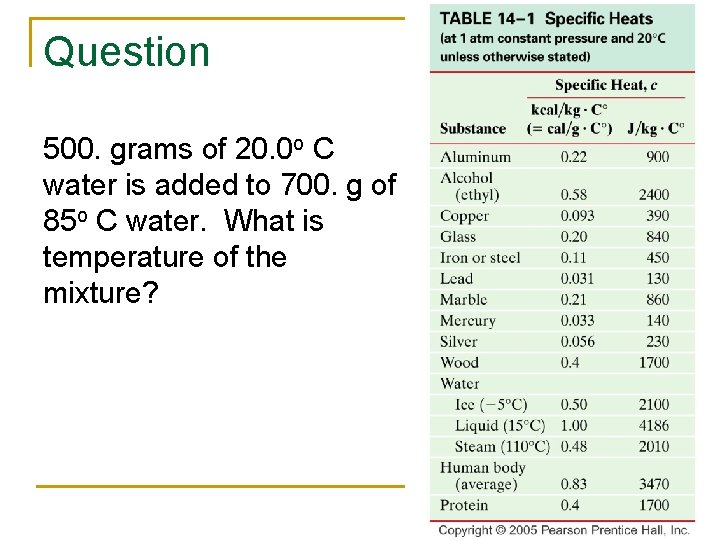
Question 500. grams of 20. 0 o C water is added to 700. g of 85 o C water. What is temperature of the mixture?

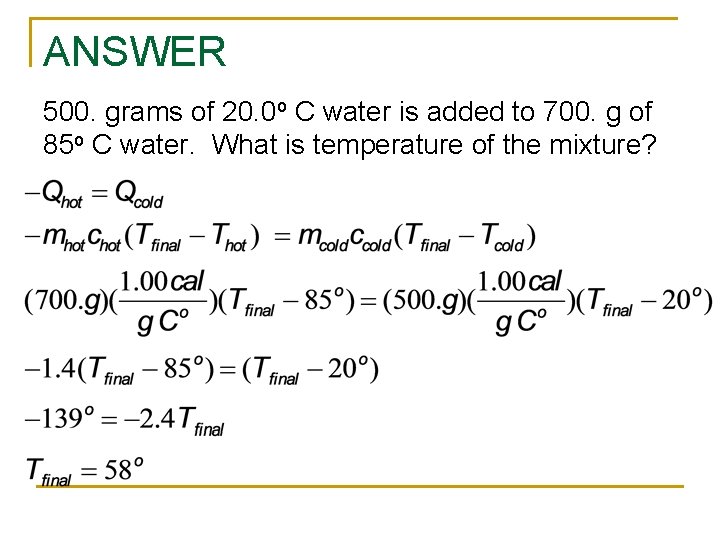
ANSWER 500. grams of 20. 0 o C water is added to 700. g of 85 o C water. What is temperature of the mixture?

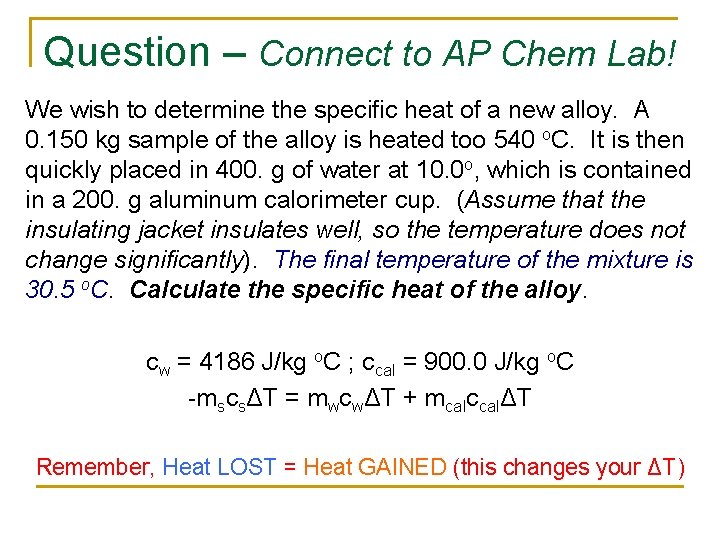
Question – Connect to AP Chem Lab! We wish to determine the specific heat of a new alloy. A 0. 150 kg sample of the alloy is heated too 540 o. C. It is then quickly placed in 400. g of water at 10. 0 o, which is contained in a 200. g aluminum calorimeter cup. (Assume that the insulating jacket insulates well, so the temperature does not change significantly). The final temperature of the mixture is 30. 5 o. C. Calculate the specific heat of the alloy. cw = 4186 J/kg o. C ; ccal = 900. 0 J/kg o. C -mscsΔT = mwcwΔT + mcalccalΔT Remember, Heat LOST = Heat GAINED (this changes your ΔT)

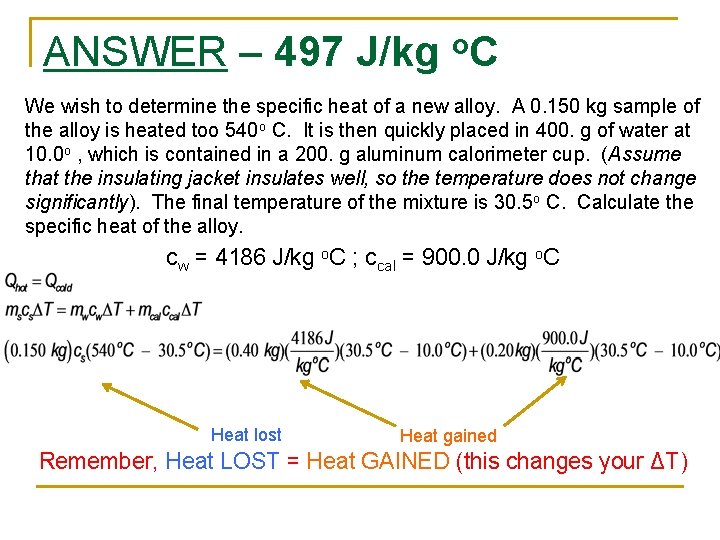
ANSWER – 497 J/kg o. C We wish to determine the specific heat of a new alloy. A 0. 150 kg sample of the alloy is heated too 540 o C. It is then quickly placed in 400. g of water at 10. 0 o , which is contained in a 200. g aluminum calorimeter cup. (Assume that the insulating jacket insulates well, so the temperature does not change significantly). The final temperature of the mixture is 30. 5 o C. Calculate the specific heat of the alloy. cw = 4186 J/kg o. C ; ccal = 900. 0 J/kg o. C Heat lost Heat gained Remember, Heat LOST = Heat GAINED (this changes your ΔT)

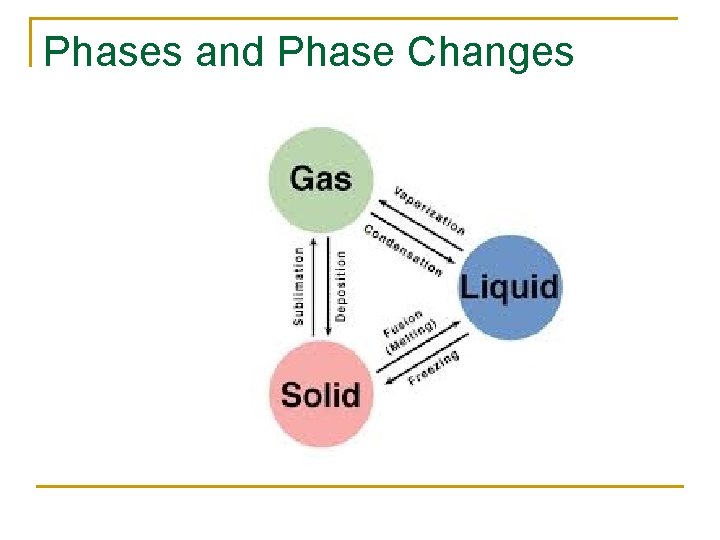
Phases and Phase Changes

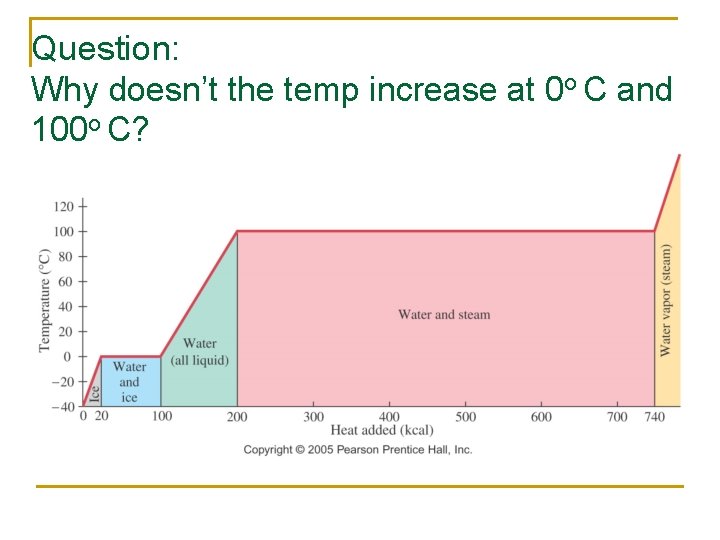
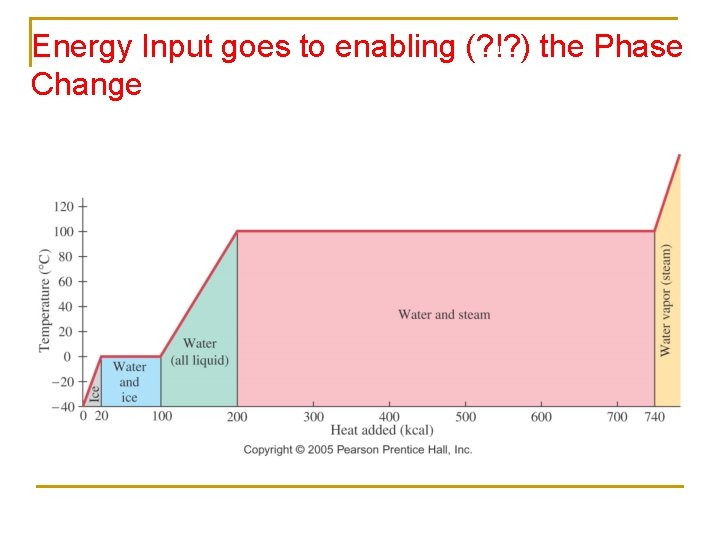
Question: Why doesn’t the temp increase at 0 o C and 100 o C?

Energy Input goes to enabling (? !? ) the Phase Change

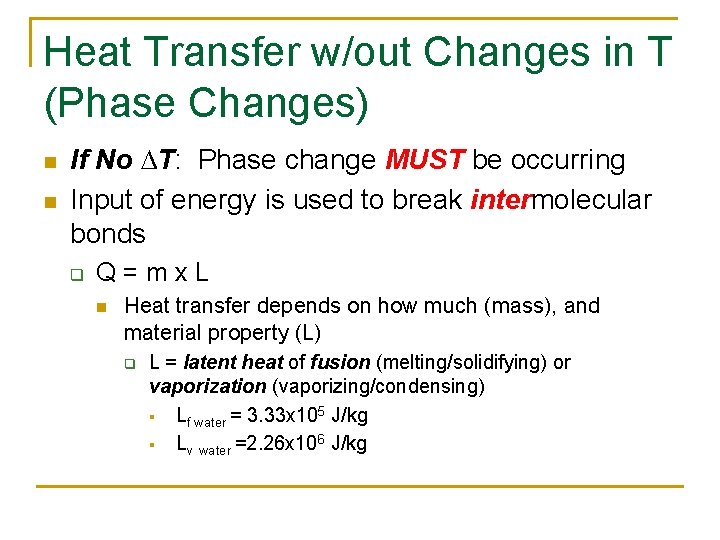
Heat Transfer w/out Changes in T (Phase Changes) n n If No ∆T: Phase change MUST be occurring Input of energy is used to break intermolecular bonds q Q=mx. L n Heat transfer depends on how much (mass), and material property (L) q L = latent heat of fusion (melting/solidifying) or vaporization (vaporizing/condensing) § Lf water = 3. 33 x 105 J/kg § Lv water =2. 26 x 106 J/kg

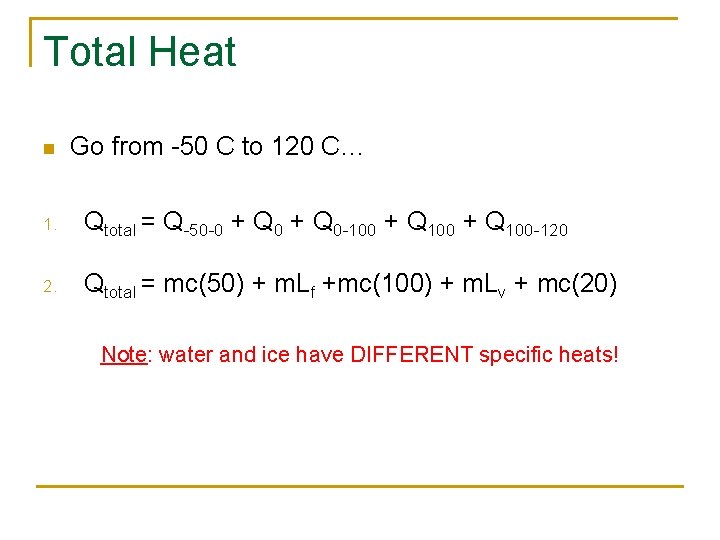
Total Heat n Go from -50 C to 120 C… 1. Qtotal = Q-50 -0 + Q 0 -100 + Q 100 -120 2. Qtotal = mc(50) + m. Lf +mc(100) + m. Lv + mc(20) Note: water and ice have DIFFERENT specific heats!

Question How much energy does a refrigerator have to remove from 1. 5 kg of water at 20. 0 o C to make ice at -12 o C. The Heat of Fusion is 3. 33 x 105 J/Kg, the specific heat of water is 4180 J/(kgo. C), the specific heat of ice is 2100 J/(kgo. C).
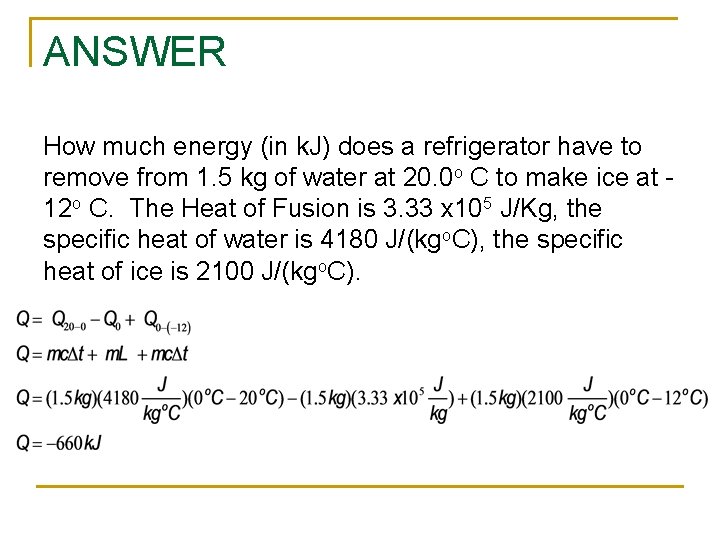
ANSWER How much energy (in k. J) does a refrigerator have to remove from 1. 5 kg of water at 20. 0 o C to make ice at 12 o C. The Heat of Fusion is 3. 33 x 105 J/Kg, the specific heat of water is 4180 J/(kgo. C), the specific heat of ice is 2100 J/(kgo. C).

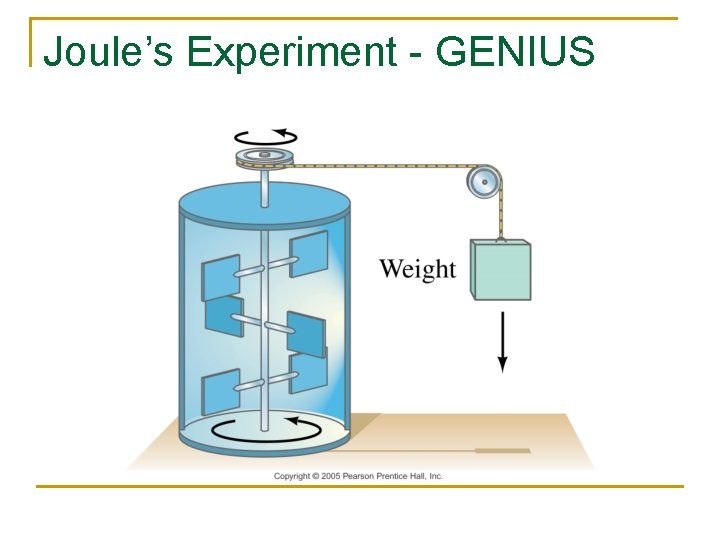
Joule’s Experiment - GENIUS

Video – Mechanical Equivalent of Heat

For the Rest of Class! n n Complete the Heat Transfer Worksheet Heat Transfer – AP Questions: MC: 10, 26, 28, 42, 43, 56, 65, 81

Now what… 1. Next Video: Ideal Gases 2. MC Question’s: 2, 5, 7, 9, 21 -23, 27, 31, 38, 44, 45, 47, 48, 51, 52, 61 -64, 73, 76, 78 3. Next Class: Quiz on Ideal Gases + Gas Law Simulation (Please bring a computer!)