Temperature Heat and Expansion Temperature Average kinetic energy








- Slides: 23

Temperature, Heat and Expansion

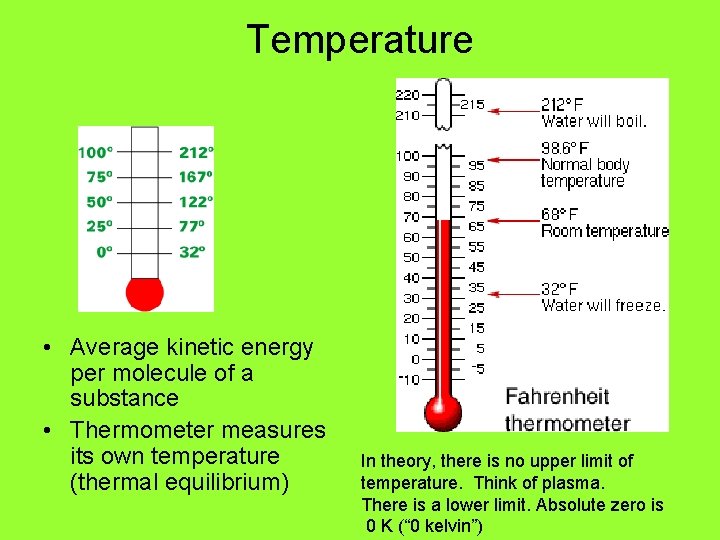
Temperature • Average kinetic energy per molecule of a substance • Thermometer measures its own temperature (thermal equilibrium) In theory, there is no upper limit of temperature. Think of plasma. There is a lower limit. Absolute zero is 0 K (“ 0 kelvin”)

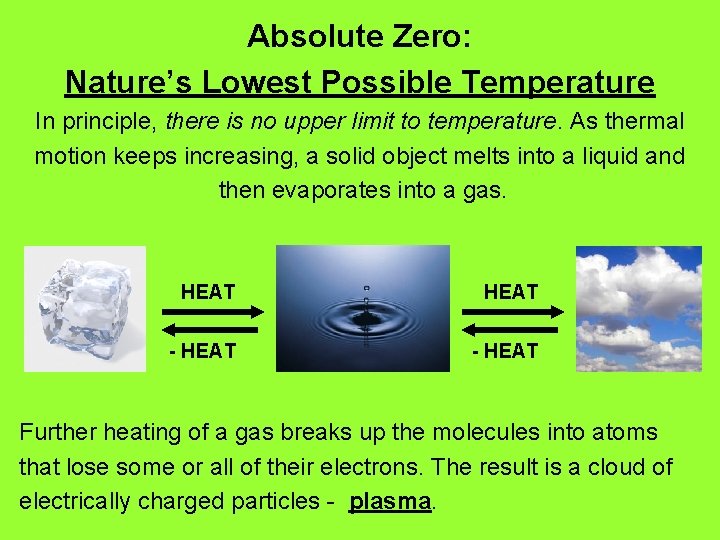
Absolute Zero: Nature’s Lowest Possible Temperature In principle, there is no upper limit to temperature. As thermal motion keeps increasing, a solid object melts into a liquid and then evaporates into a gas. HEAT - HEAT Further heating of a gas breaks up the molecules into atoms that lose some or all of their electrons. The result is a cloud of electrically charged particles - plasma.

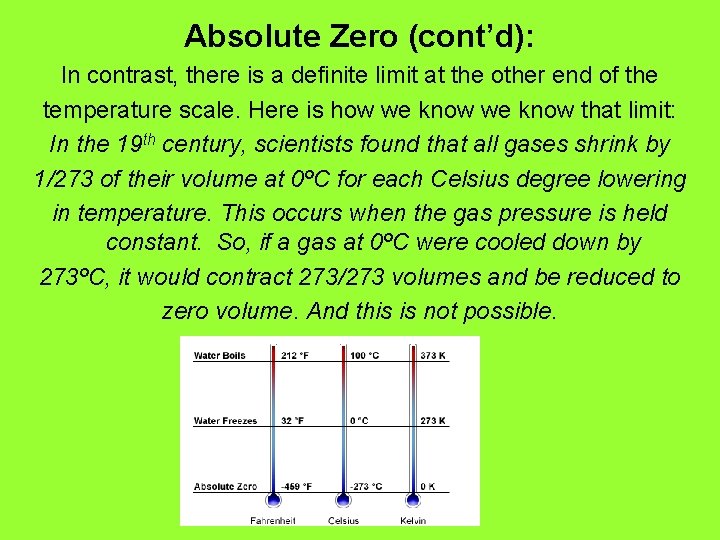
Absolute Zero (cont’d): In contrast, there is a definite limit at the other end of the temperature scale. Here is how we know that limit: In the 19 th century, scientists found that all gases shrink by 1/273 of their volume at 0ºC for each Celsius degree lowering in temperature. This occurs when the gas pressure is held constant. So, if a gas at 0ºC were cooled down by 273ºC, it would contract 273/273 volumes and be reduced to zero volume. And this is not possible.

Absolute Zero (cont’d): So, the lower limit to temperature is -273ºC, or Absolute Zero. Not absolute zero, but definitely very cold.

Temperature and Kinetic Energy • Which has greater temperature? • Which has more kinetic energy?

Heat • Energy transferred from one thing to another because of a temperature difference • Thermal energy in transit • Flows from high temperature substance to low temperature substance • Objects contain kinetic energy and potential energy but not heat. • Internal energy

Concept Check Distinguish between temperature and heat. TEMPERATURE: a measure, in degrees Celsius, Kelvin, or Fahrenheit of the average kinetic energy per molecule in an object HEAT: The thermal energy that flows from an object at higher temperature to one at lower temperature.

Measurement of Heat • Can be measured in joules • 4. 184 J = 1 cal • calorie: amount of heat required to change the temperature of 1 gram of water by 1 degree Celsius 1 Calorie = 1000 calories

The Laws of Thermodynamics (There are three. ) 1) The First Law of Thermodynamics: Whenever heat flows into or out of a system, the gain or loss of thermal energy equals the amount of heat transferred. 2) The Second Law of Thermodynamics: Heat never spontaneously flows from a cold substance to a hot substance. 3) The Third Law of Thermodynamics No system can reach absolute zero.

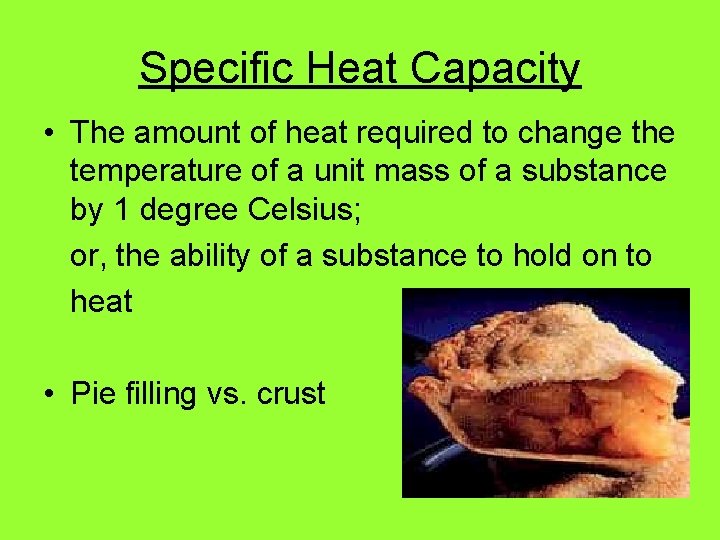
Specific Heat Capacity • The amount of heat required to change the temperature of a unit mass of a substance by 1 degree Celsius; or, the ability of a substance to hold on to heat • Pie filling vs. crust

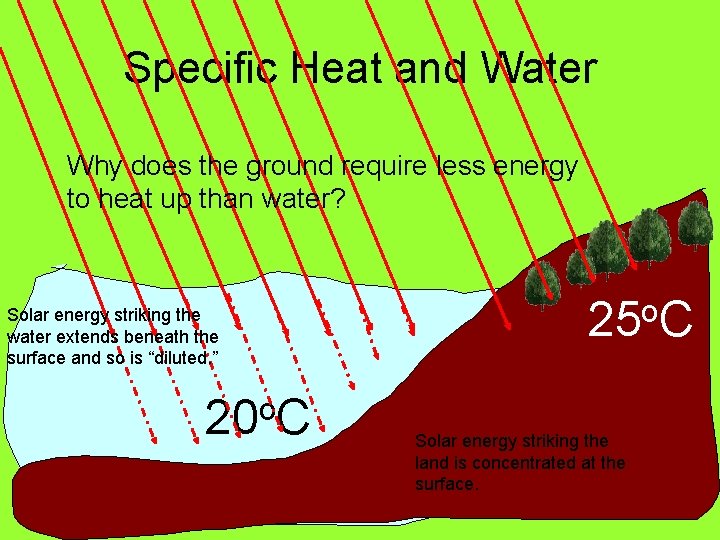
Specific Heat and Water Why does the ground require less energy to heat up than water? Solar energy striking the water extends beneath the surface and so is “diluted. ” o 20 C 25 o. C Solar energy striking the land is concentrated at the surface.

Specific Heat and Water • Water has a much higher capacity for storing energy than most other substances. • Water has a high specific heat capacity. • A lot of heat energy is needed to change the temperature of the water. • Water absorbs a great quantity of heat for small rises in temperature. Water also takes longer to cool.

Geography’s Role • Living in DC vs. San Francisco

Geography’s Role • Water’s high specific heat changes the world’s climate. • Example: Gulf stream current carries warm water NE from the Caribbean

Concept Check 1. Desert sand is very hot in the day and very cool at night. What does this tell you about its specific heat? 2. Why is it important to protect water pipes so they don’t freeze in the winter? 3. Adding the same amount of heat to two different objects (of the same mass) does not necessarily produce the same increase in temperature. Why not?

Thermal Expansion Most substances expand when heated and contract when cooled. Can you name an exception? Rockers and Expansion joints

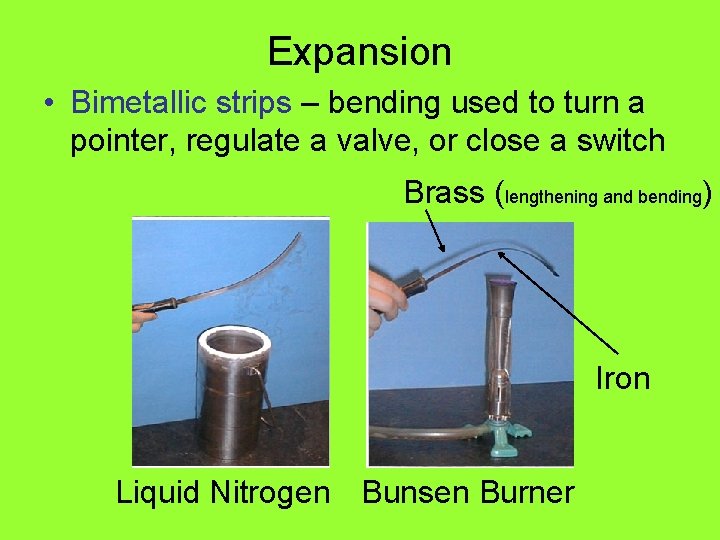
Expansion • Bimetallic strips – bending used to turn a pointer, regulate a valve, or close a switch Brass (lengthening and bending) Iron Liquid Nitrogen Bunsen Burner

Bimettalic Strips • Used in electric toasters, oven thermometers, refrigerators, and thermostats

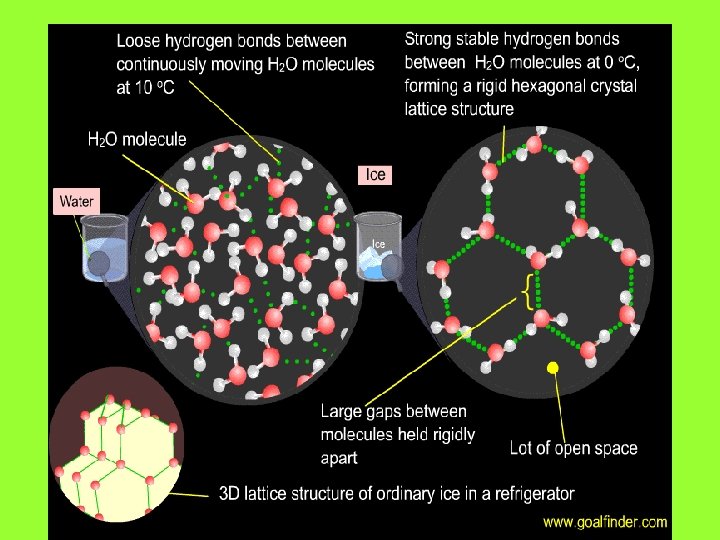
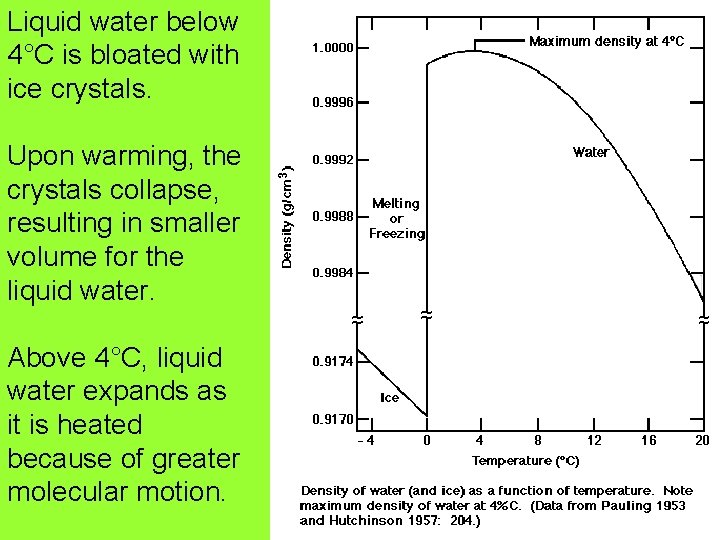
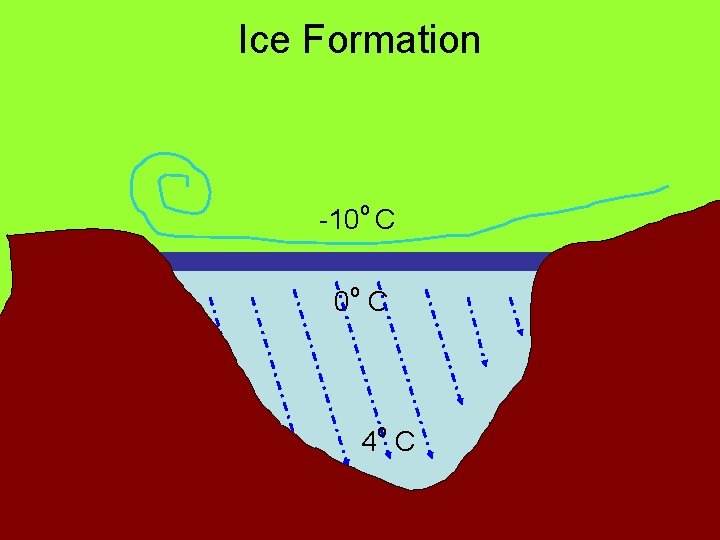
Expansion of Water expands when heated. But it doesn’t expand between 0°C and 4°C. Why?


Liquid water below 4°C is bloated with ice crystals. Upon warming, the crystals collapse, resulting in smaller volume for the liquid water. Above 4°C, liquid water expands as it is heated because of greater molecular motion.

Ice Formation o -10 C 0 o C 4 o C