Temperature Heat and Expansion Chapter 21 Temperature n














- Slides: 21

Temperature, Heat, and Expansion Chapter 21

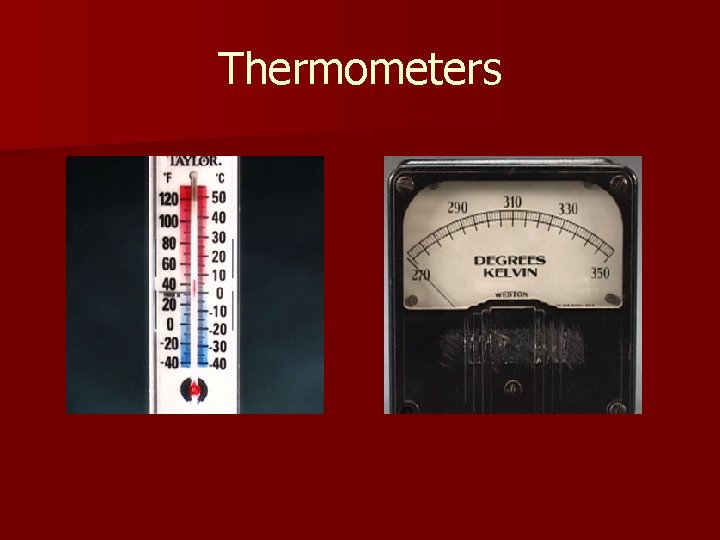
Temperature n Temperature – the quantity that tells how hot or cold something is compared with a standard n A common thermometer measures temperature by showing the expansion and contraction of a liquid in a glass tube using a scale n Celsius Scale – most widely used temperature scale, 0ºC is the point at which water freezes and 100ºC the point at which water boils, the gap between is divided into 100 equal parts

Temperature n Fahrenheit Scale – commonly used in the U. S. , has 180ºF between freezing and boiling (32ºF and 212º) n Kelvin Scale – used in scientific research, degrees are the same size as Celsius + 273º, denoted K n Absolute Zero – the lowest possible temperature on the Kelvin scale, substance has no kinetic energy

Thermometers

Scale Comparison

Temperature and Kinetic Energy n In an ideal gas, temperature is proportional to the average kinetic energy n The heat that you feel when you touch a hot surface is the kinetic energy transferred by molecules in the surface to molecules in your fingers n Temperature is not a measure of the total kinetic energy

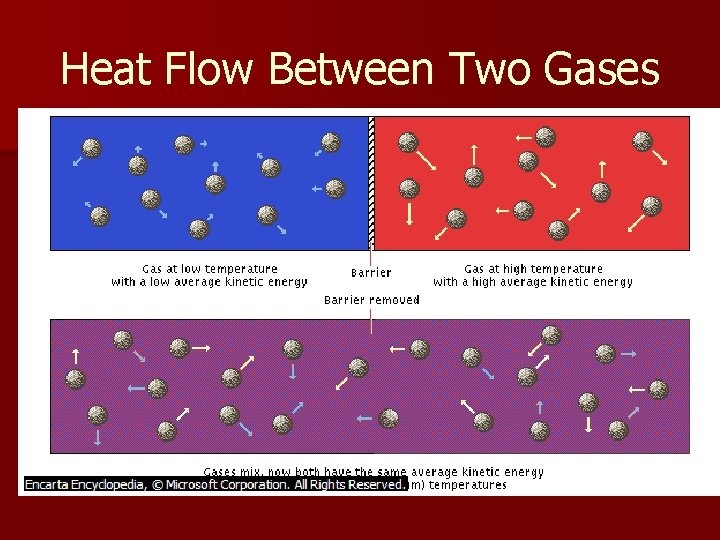
Heat n n n Heat – the energy that transfers from one object to another because of a temperature difference between them Matter does not contain heat, but contains energy in several forms Heat is energy in transit Internal Energy – the energy resulting from heat flow When heat flows from one object or substance to another it is in contact with, the objects are said to be in thermal contact Heat flows from the higher-temperature substance into the lower-temperature substance

Heat Flow Between Two Gases

Thermal Equilibrium n Thermal Equilibrium – objects in thermal contact with each other reach the same temperature, no heat flows between them n When reading a thermometer, we wait until thermometer has reached thermal equilibrium with the object we want the temperature of

Thermal Equilibrium

Internal Energy n Internal Energy – the total of all energies inside a substance n A substance does not contain heat – it contains internal energy n When a substance takes in or gives off heat, any of these energies can change

Internal Energy

Measurement of Heat n n n To quantify heat, we have to specify the mass and kind of substance affected Calorie – the most commonly used unit for heat; the amount of heat required to raise the temperature of 1 gram of water by 1ºC Kilocalorie – the heat required to raise 1 kilogram of water by 1ºC (1000 calories) The heat unit for rating foods is actually the kilocalorie (to distinguish from calorie, it is often written as Calorie) Remember that a calorie is a measure of ENERGY! The relationship between calories and joules is: 1 calorie = 4. 184 Joules

Calories

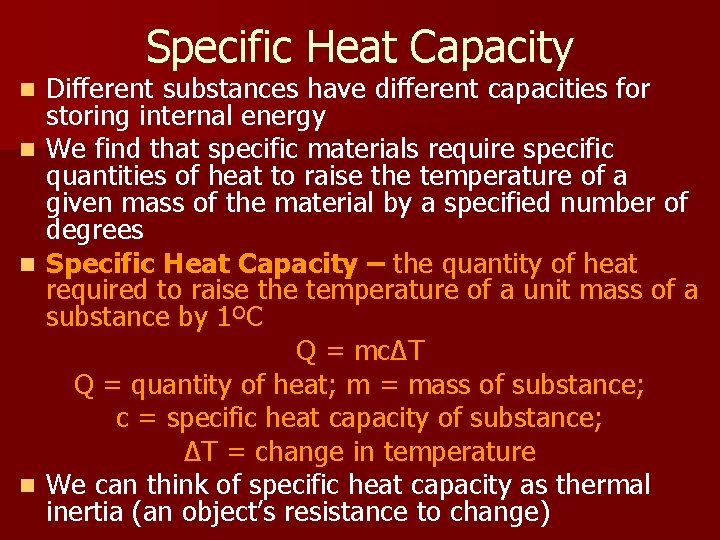
Specific Heat Capacity Different substances have different capacities for storing internal energy n We find that specific materials require specific quantities of heat to raise the temperature of a given mass of the material by a specified number of degrees n Specific Heat Capacity – the quantity of heat required to raise the temperature of a unit mass of a substance by 1ºC Q = mcΔT Q = quantity of heat; m = mass of substance; c = specific heat capacity of substance; ΔT = change in temperature n We can think of specific heat capacity as thermal inertia (an object’s resistance to change) n

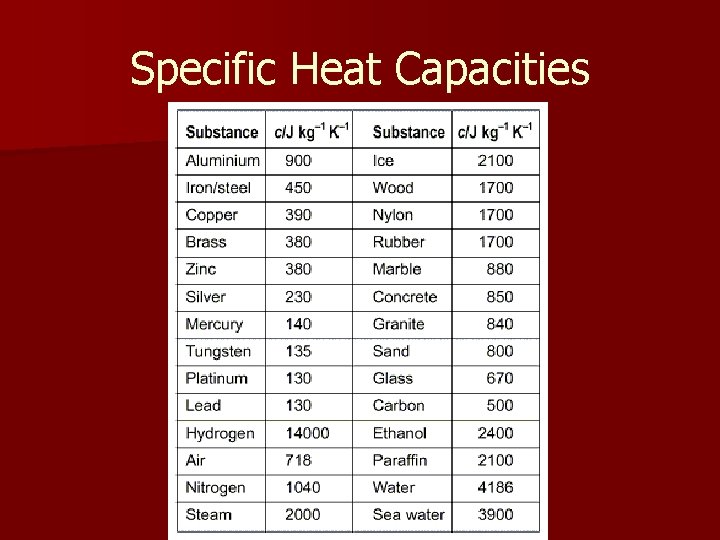
Specific Heat Capacities

Thermal Expansion When the temperature of a substance increases, the molecules “jiggle” faster and move further apart, causing an expansion of the substance n Gases generally expand contract more than liquids, which expand contract more than solids n In concrete sidewalks and highways this expansion and contraction is taken into account when it is being built. The surface is laid down in small sections with a gap in between, that is usually filled with a substance such as tar. n

Thermal Expansion Joint

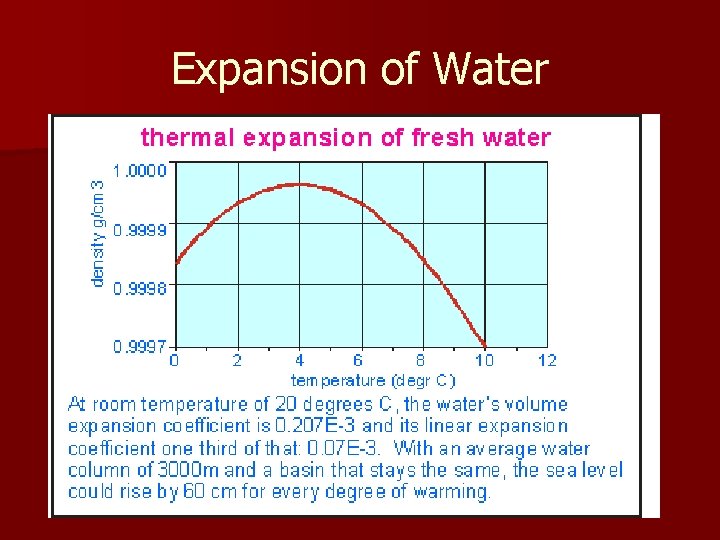
Expansion of Water n Almost all liquids will expand when they are heated, ice-cold water instead contracts to go from ice to a liquid n When the water reaches a temperature of 4ºC, it will stop contracting and begin expanding n This has to do with the crystal structure of water, its solid state has an open structure that takes up more volume and is therefore less dense

Expansion of Water

Assignment n Read Chapter 21 (pg. 307 -321) n Do Chapter 21 #19 -39 (pg. 323 -324) n Do Appendix F #1 -7 (pg. 680)