Temperature Fahrenheit Celsius and Kelvin Temperature vs Heat







![Temperature Kelvin Degrees Celsius Peak emittance wavelength[65] of black-body radiation 0 K − 273. Temperature Kelvin Degrees Celsius Peak emittance wavelength[65] of black-body radiation 0 K − 273.](https://slidetodoc.com/presentation_image_h2/adba032614f0c8df4efa989809c20fcd/image-11.jpg)




- Slides: 18

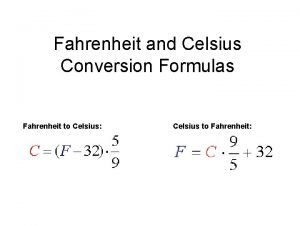
Temperature: Fahrenheit, Celsius, and Kelvin

Temperature vs. Heat With your neighbor, try to distinguish between temperature and heat. (Qualitatively and Quantitatively)

Thermal Energy (Heat) & Temperature • How would you describe the temperature of a steaming cup of coffee? • If you said it is “hot” do you mean: A) It has high temperature Or B) It has a large amount of thermal energy?

• Let’s think about Temperature and Thermal Energy and see if there is a difference.

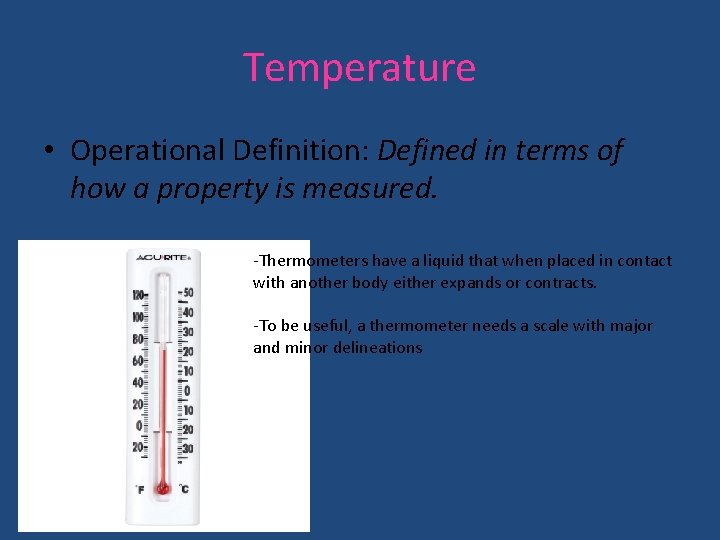
Temperature • Operational Definition: Defined in terms of how a property is measured. -Thermometers have a liquid that when placed in contact with another body either expands or contracts. -To be useful, a thermometer needs a scale with major and minor delineations

Celsius • 1742 Swedish astronomer, Anders Celsius used a mercury thermometer and defined his scale in terms of critical points of pure water. Advantages: -Reproducible -Scale of 10 Disadvantages: -Arbitrary zero value -Negative numbers CONVERSION

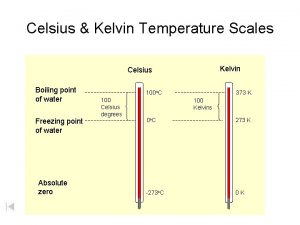
Kelvin • 1848, British scientist, William Thomson Lord Kelvin developed a scale that relies on the average kinetic energy of atoms. Advantages: -Absolute scale -Empirical -No Negatives -Still a scale of 10 CONVERSION TK = TC + 273

Temperature • Roughly speaking, temperature is a comparative measure of hot and cold • Kelvin is based on measuring the average kinetic energy of atoms in a sample…

Thermal Energy • The sum of the kinetic and potential energies of the atoms/molecules in a body. Thermal Energy is also referred to as INTERNAL Energy.

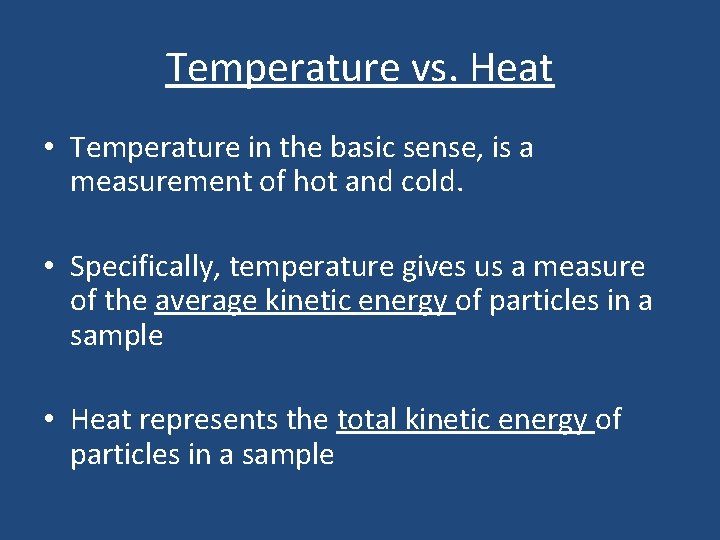
Temperature vs. Heat • Temperature in the basic sense, is a measurement of hot and cold. • Specifically, temperature gives us a measure of the average kinetic energy of particles in a sample • Heat represents the total kinetic energy of particles in a sample
![Temperature Kelvin Degrees Celsius Peak emittance wavelength65 of blackbody radiation 0 K 273 Temperature Kelvin Degrees Celsius Peak emittance wavelength[65] of black-body radiation 0 K − 273.](https://slidetodoc.com/presentation_image_h2/adba032614f0c8df4efa989809c20fcd/image-11.jpg)
Temperature Kelvin Degrees Celsius Peak emittance wavelength[65] of black-body radiation 0 K − 273. 15 °C cannot be defined 100 p. K − 273. 14999900 °C 29, 000 km 450 p. K − 273. 14999999955 °C 6, 400 km 0. 001 K − 273. 149 °C 273. 16 K 0. 01 °C Water's boiling point[A] 373. 1339 K 99. 9839 °C Incandescent lamp[B] 2500 K ≈2, 200 °C Sun's visible surface[D][69] 5, 778 K 5, 505 °C 28 k. K 28, 000 °C 16 MK 16 million °C 350 MK 350 million °C 2 GK 2 billion °C 3 GK 3 billion °C 350 GK 350 billion °C 1 TK 1 trillion °C 10 TK 10 trillion °C 1. 417× 10 32 K 1. 417× 10 32 °C Absolute zero (precisely by definition) Coldest temperature achieved[66] Coldest Bose–Einstein condensate[67] One millikelvin (precisely by definition) Water's triple point (precisely by definition) Lightning bolt's channel[E] Sun's core[E] Thermonuclear weapon (peak temperature)[E][70] Sandia National Labs' Z machine[E][71] Core of a high-mass star on its last day[E][72] Merging binary neutron star system[E][73] Relativistic Heavy Ion Collider[E][74] CERN's proton vs nucleus collisions[E][75] Universe 5. 391× 10 − 44 s after the Big Bang[E] 2. 89777 m (radio, FM band)[68] 10, 608. 3 nm (long wavelength I. R. ) 7, 766. 03 nm (mid wavelength I. R. ) 1, 160 nm (near infrared)[C] 501. 5 nm (green-blue light) 100 nm (far ultraviolet light) 0. 18 nm (X-rays) 8. 3× 10− 3 nm (gamma rays) 1. 4× 10− 3 nm (gamma rays)[F] 1× 10− 3 nm (gamma rays) 8× 10− 6 nm (gamma rays) 3× 10− 7 nm (gamma rays) 1. 616× 10 − 27 nm (Planck Length)[76]

Physical Properties that Depend on Temperature

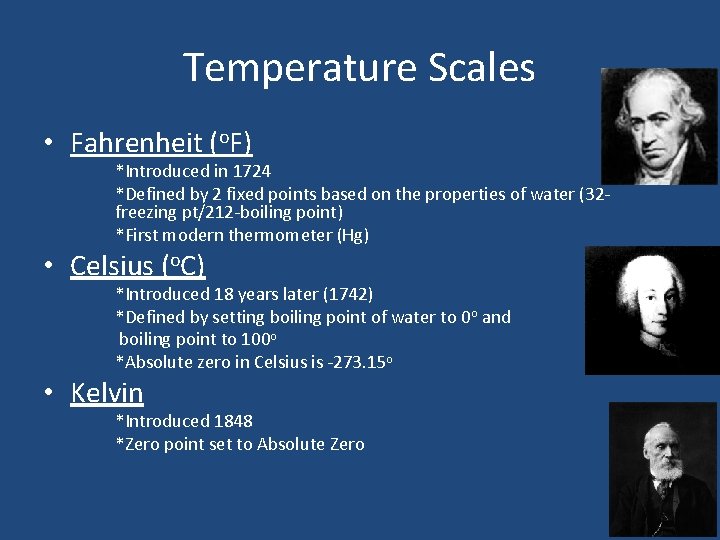
Temperature Scales • Fahrenheit (o. F) • *Introduced in 1724 *Defined by 2 fixed points based on the properties of water (32 freezing pt/212 -boiling point) *First modern thermometer (Hg) Celsius (o. C) *Introduced 18 years later (1742) *Defined by setting boiling point of water to 0 o and boiling point to 100 o *Absolute zero in Celsius is -273. 15 o • Kelvin *Introduced 1848 *Zero point set to Absolute Zero

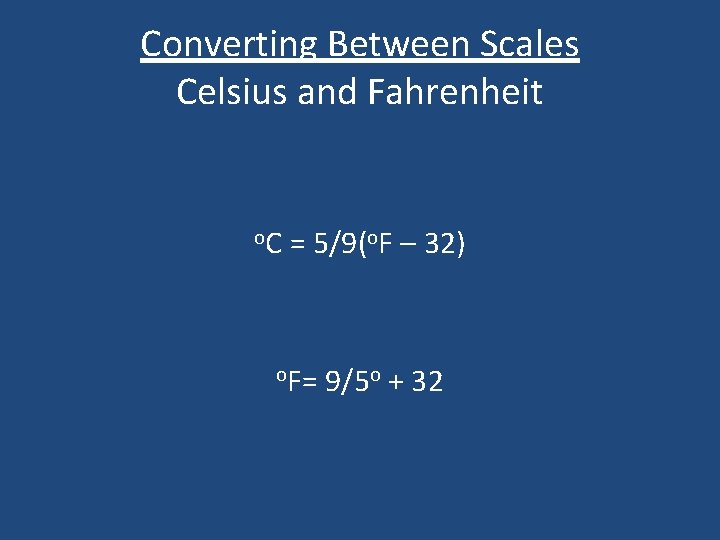
Converting Between Scales Celsius and Fahrenheit o. C = 5/9(o. F – 32) o. F= 9/5 o + 32

Converting Between Scales Celsius and Kelvin K= o. C + 273

Practice • Convert 32 o. F into Celsius (Proof of Concept)

Practice • Convert 32 o. C into K

Practice • Convert 580 o. F into K
 25celsius to fahrenheit
25celsius to fahrenheit Gabriel fahrenheit and anders celsius
Gabriel fahrenheit and anders celsius -40f to celsius
-40f to celsius 451 fahrenheit to kelvin
451 fahrenheit to kelvin What is 12 celsius in fahrenheit
What is 12 celsius in fahrenheit 451 degrees fahrenheit is the temperature at which
451 degrees fahrenheit is the temperature at which Decimal system of measurement
Decimal system of measurement How to read a weather thermometer
How to read a weather thermometer Flowchart to convert fahrenheit to celsius
Flowchart to convert fahrenheit to celsius 70o fahrenheit to celsius
70o fahrenheit to celsius Freezing fahrenheit 32
Freezing fahrenheit 32 Fahrenheit to celsius formula in c
Fahrenheit to celsius formula in c 350 fahrenheit to celsius
350 fahrenheit to celsius 320 fahrenheit to celsius
320 fahrenheit to celsius Dsp tms
Dsp tms 320 fahrenheit to celsius
320 fahrenheit to celsius Front wheel.drive tire rotation
Front wheel.drive tire rotation Transformare fahrenheit in celsius
Transformare fahrenheit in celsius Danger zone food
Danger zone food