Temperature changes Hydrochloric acid 15 cm 3 2

- Slides: 12

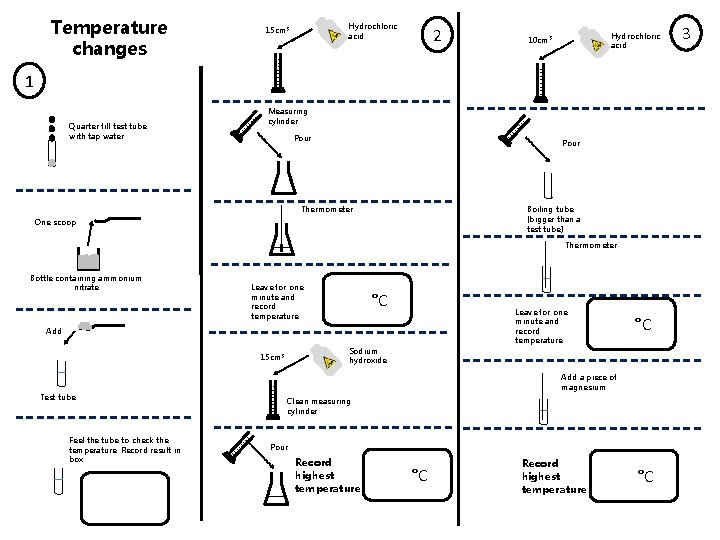
Temperature changes Hydrochloric acid 15 cm 3 2 Hydrochloric acid 10 cm 3 1 Quarter fill test tube with tap water Measuring cylinder Pour Thermometer Boiling tube (bigger than a test tube) One scoop Thermometer Bottle containing ammonium nitrate Leave for one minute and record temperature °C Leave for one minute and record temperature Add Sodium hydroxide 15 cm 3 Test tube Feel the tube to check the temperature. Record result in box °C Add a piece of magnesium Clean measuring cylinder Pour Record highest temperature °C 3

Complete in your book 1. For experiment 1 a) What did you observe? b) When the ammonium nitrate is added to the water, c) d) e) f) 2. For experiment 2 a) By how much did the temperature change in the b) c) d) e) f) 3. what happens to the temperature? Has a chemical reaction occurred? If the mass of the test tube was 15 g, ammonium nitrate 3. 1 g and water 4 g, what would the mass of the test tube and everything in it be at the beginning of the reaction? Would the mass have increased, decreased or stayed the same? Explain your answer to e reaction? When sodium hydroxide and hydrochloric acid react together they make sodium chloride and water. Write a word equation for this reaction. What is the formula for water? What are the reactants and what are the products in this reaction? Water is a molecular substance. What does this mean? Sodium chloride is safe to eat but sodium and chlorine are both toxic. Explain why this is. For experiment 3 a) What did you observe during this reaction? b) How do you know a chemical reaction took place? c) Magnesium reacts with hydrochloric acid to form d) e) f) magnesium chloride and hydrogen. Write a word equation for this reaction. Identify all elements and compounds in the equation The symbol equation for this reaction is below. Copy it into your exercise book and balance it. Mg + HCl Mg. Cl 2 + H 2 During this reaction, the overall mass decreases. Explain why this happens.

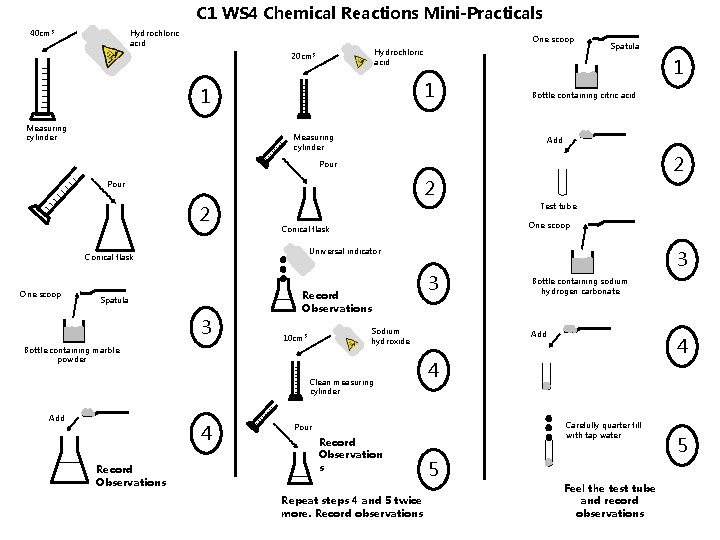
C 1 WS 4 Chemical Reactions Mini-Practicals Hydrochloric acid 40 cm 3 One scoop Hydrochloric acid 20 cm 3 1 1 Measuring cylinder Spatula 1 Bottle containing citric acid Add 2 Pour 2 Test tube Universal indicator Conical flask One scoop Conical flask Spatula 3 Record Observations Sodium hydroxide 10 cm 3 Bottle containing marble powder Clean measuring cylinder Add 4 Record Observations 3 Repeat steps 4 and 5 twice more. Record observations Bottle containing sodium hydrogen carbonate Add 4 4 Carefully quarter fill with tap water Pour Record Observation s 3 5 Feel the test tube and record observations 5

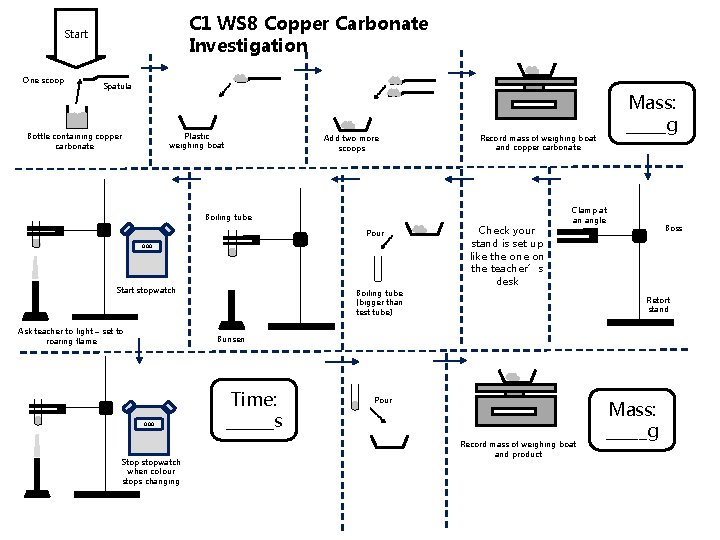
C 1 WS 8 Copper Carbonate Investigation Start One scoop Spatula Plastic weighing boat Bottle containing copper carbonate Add two more scoops Record mass of weighing boat and copper carbonate Boiling tube Pour 0. 00 Start stopwatch Ask teacher to light – set to roaring flame Boiling tube (bigger than test tube) Mass: _____g Check your stand is set up like the on the teacher’s desk Clamp at an angle Boss Retort stand Bunsen 0. 00 Stop stopwatch when colour stops changing Time: ______s Pour Record mass of weighing boat and product Mass: _____g

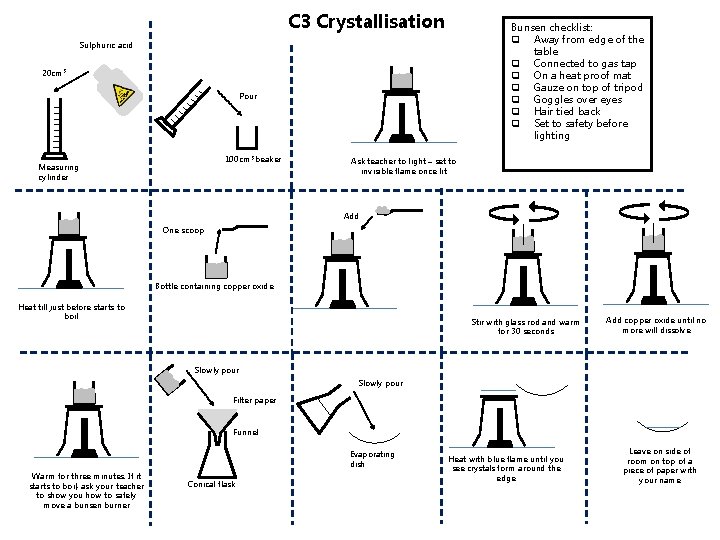
C 3 Crystallisation Bunsen checklist: q Away from edge of the table q Connected to gas tap q On a heat proof mat q Gauze on top of tripod q Goggles over eyes q Hair tied back q Set to safety before lighting Sulphuric acid 20 cm 3 Pour 100 cm 3 beaker Measuring cylinder Ask teacher to light – set to invisible flame once lit Add One scoop Bottle containing copper oxide Heat till just before starts to boil Stir with glass rod and warm for 30 seconds Add copper oxide until no more will dissolve Slowly pour Filter paper Funnel Evaporating dish Warm for three minutes. If it starts to boil, ask your teacher to show you how to safely move a bunsen burner Conical flask Heat with blue flame until you see crystals form around the edge Leave on side of room on top of a piece of paper with your name

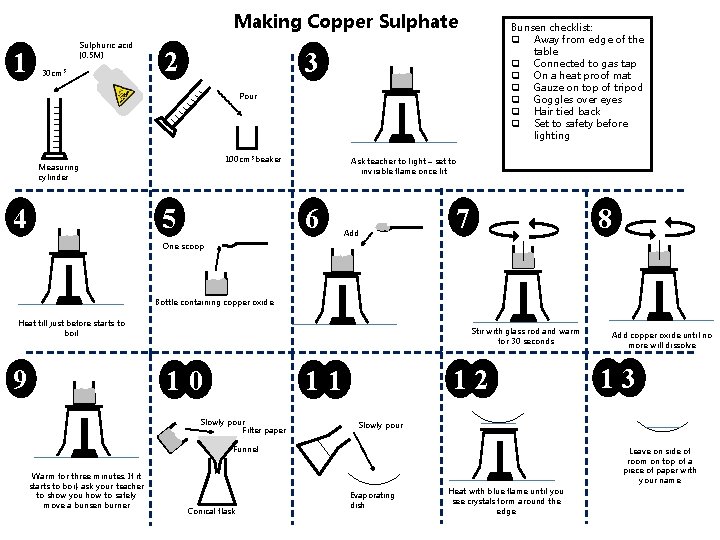
Making Copper Sulphate 1 Sulphuric acid (0. 5 M) 30 cm 3 2 Bunsen checklist: q Away from edge of the table q Connected to gas tap q On a heat proof mat q Gauze on top of tripod q Goggles over eyes q Hair tied back q Set to safety before lighting 3 Pour 100 cm 3 beaker Measuring cylinder 4 5 Ask teacher to light – set to invisible flame once lit 6 Add 7 8 One scoop Bottle containing copper oxide Heat till just before starts to boil 9 Stir with glass rod and warm for 30 seconds 10 12 11 Slowly pour Filter paper Conical flask 13 Slowly pour Funnel Warm for three minutes. If it starts to boil, ask your teacher to show you how to safely move a bunsen burner Add copper oxide until no more will dissolve Leave on side of room on top of a piece of paper with your name Evaporating dish Heat with blue flame until you see crystals form around the edge

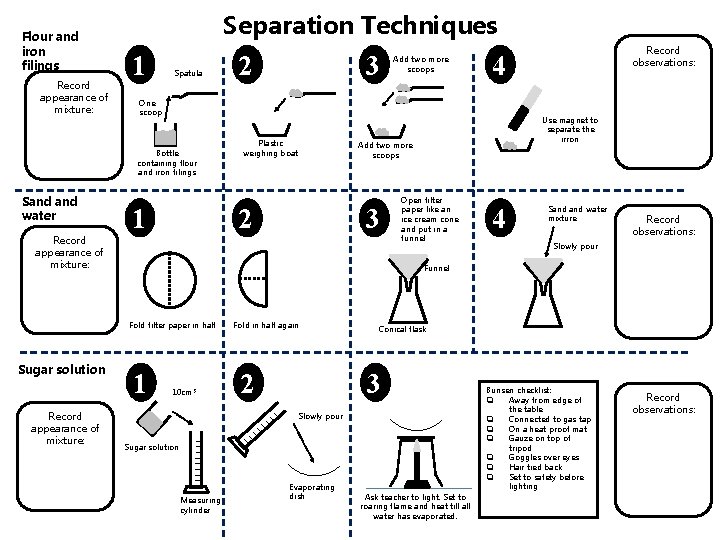
Flour and iron filings Record appearance of mixture: Separation Techniques 1 Spatula Record appearance of mixture: 1 Record appearance of mixture: Plastic weighing boat 2 4 Use magnet to separate the irron Add two more scoops 3 Record observations: Open filter paper like an ice cream cone and put in a funnel 4 Sand water mixture Record observations: Slowly pour Funnel Fold filter paper in half Sugar solution 3 One scoop Bottle containing flour and iron filings Sand water 2 Add two more scoops 1 10 cm 3 Fold in half again 2 Conical flask 3 Slowly pour Sugar solution Measuring cylinder Evaporating dish Ask teacher to light. Set to roaring flame and heat till all water has evaporated. Bunsen checklist: q Away from edge of the table q Connected to gas tap q On a heat proof mat q Gauze on top of tripod q Goggles over eyes q Hair tied back q Set to safety before lighting Record observations:

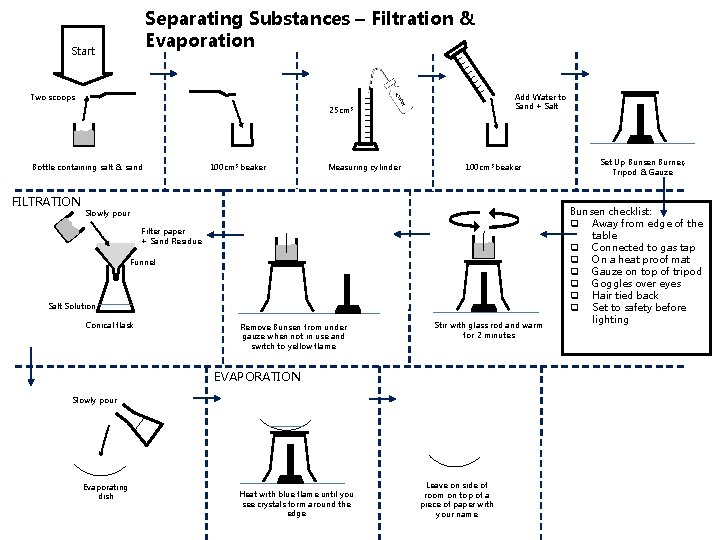
Separating Substances – Filtration & Evaporation Start Add Water to Sand + Salt Two scoops 25 cm 3 Bottle containing salt & sand FILTRATION 100 cm 3 beaker Measuring cylinder 100 cm 3 beaker Slowly pour Filter paper + Sand Residue Funnel Salt Solution Conical flask Remove Bunsen from under gauze when not in use and switch to yellow flame Stir with glass rod and warm for 2 minutes EVAPORATION Slowly pour Evaporating dish Heat with blue flame until you see crystals form around the edge Leave on side of room on top of a piece of paper with your name Set Up Bunsen Burner, Tripod & Gauze Bunsen checklist: q Away from edge of the table q Connected to gas tap q On a heat proof mat q Gauze on top of tripod q Goggles over eyes q Hair tied back q Set to safety before lighting

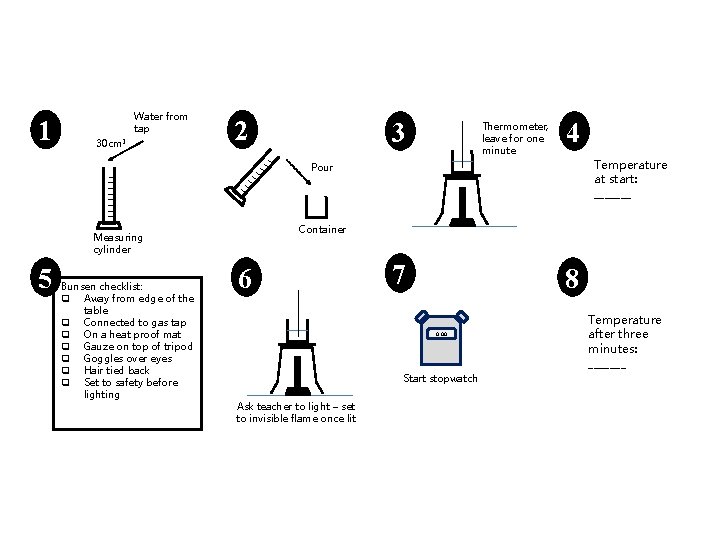
1 Water from tap 30 cm 3 2 3 Thermometer, leave for one minute 4 Temperature at start: _______ Pour Container Measuring cylinder 5 Bunsen checklist: q Away from edge of the table q Connected to gas tap q On a heat proof mat q Gauze on top of tripod q Goggles over eyes q Hair tied back q Set to safety before lighting 6 7 8 0. 00 Start stopwatch Ask teacher to light – set to invisible flame once lit Temperature after three minutes: _______

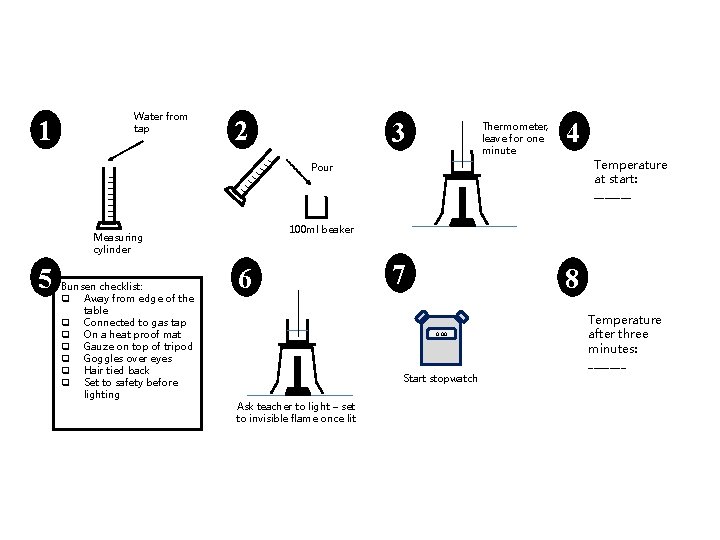
1 Water from tap 2 3 Thermometer, leave for one minute 4 Temperature at start: _______ Pour 100 ml beaker Measuring cylinder 5 Bunsen checklist: q Away from edge of the table q Connected to gas tap q On a heat proof mat q Gauze on top of tripod q Goggles over eyes q Hair tied back q Set to safety before lighting 6 7 8 0. 00 Start stopwatch Ask teacher to light – set to invisible flame once lit Temperature after three minutes: _______

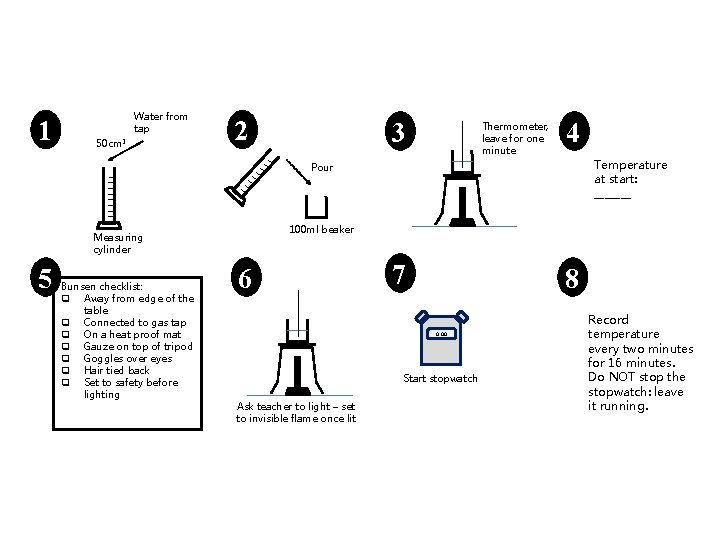
1 Water from tap 50 cm 3 2 3 Thermometer, leave for one minute 4 Temperature at start: _______ Pour 100 ml beaker Measuring cylinder 5 Bunsen checklist: q Away from edge of the table q Connected to gas tap q On a heat proof mat q Gauze on top of tripod q Goggles over eyes q Hair tied back q Set to safety before lighting 6 7 8 0. 00 Start stopwatch Ask teacher to light – set to invisible flame once lit Record temperature every two minutes for 16 minutes. Do NOT stop the stopwatch: leave it running.

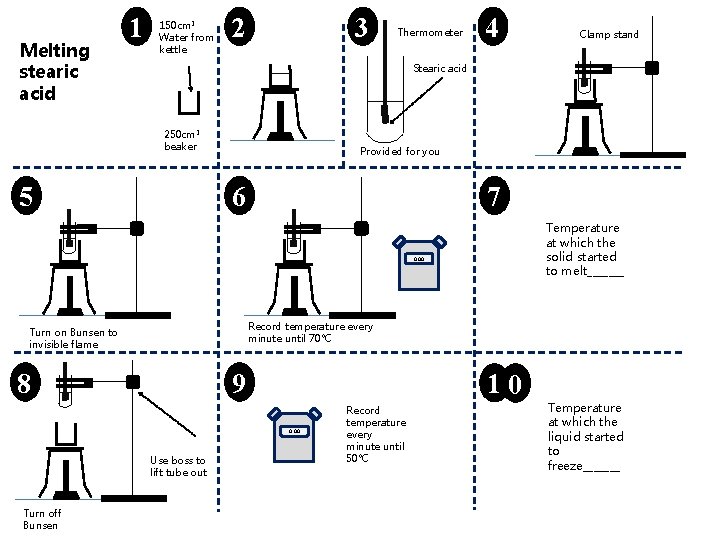
Melting stearic acid 1 150 cm 3 Water from kettle 2 3 Thermometer 4 Stearic acid 250 cm 3 beaker 5 Provided for you 6 7 Temperature at which the solid started to melt_______ 0. 00 Record temperature every minute until 70°C Turn on Bunsen to invisible flame 8 9 10 0. 00 Use boss to lift tube out Turn off Bunsen Clamp stand Record temperature every minute until 50°C Temperature at which the liquid started to freeze_______