Temperature and Volume Relationship of Gases Charless Law








- Slides: 14

Temperature and Volume Relationship of Gases (Charles’s Law) Temperature and Pressure Relationship of Gases (Gay Lussac’s Law) A. 10/A. 11 In text

• Using the data you gathered in investigation A. 9 fill in the table: Temp Volume (o. C) (in mm) 90 o 8. 5 mm 80 o 7. 9 mm 70 o 7. 3 mm Based on A. 9 and A. 1 investigations: what is the relationship between Temperature and Volume in gases?

A. 1 Investigation 2: Balloons in Water of Differing Temperatures • What happened to the volume of the balloon in hot water: • What happened to the volume of the balloon in ice water:


• Summarize the relationship between pressure and volume for a gas at a constant temperature. • What is the quantitative way to summarize this relationship? (the mathematical formula? )

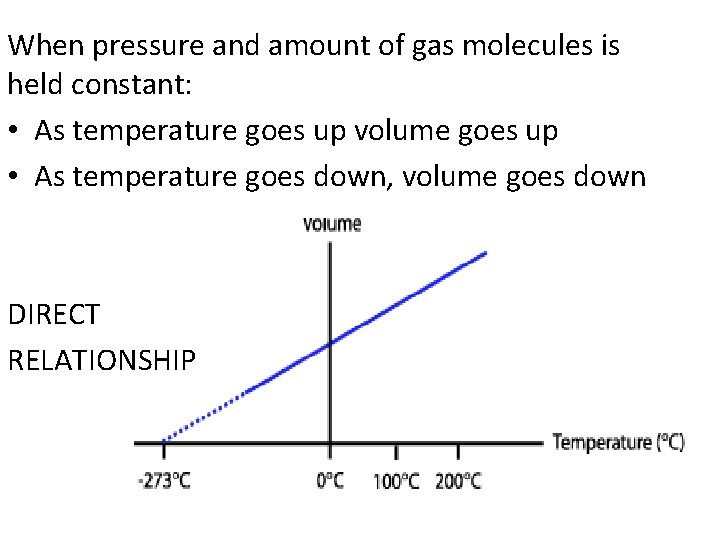
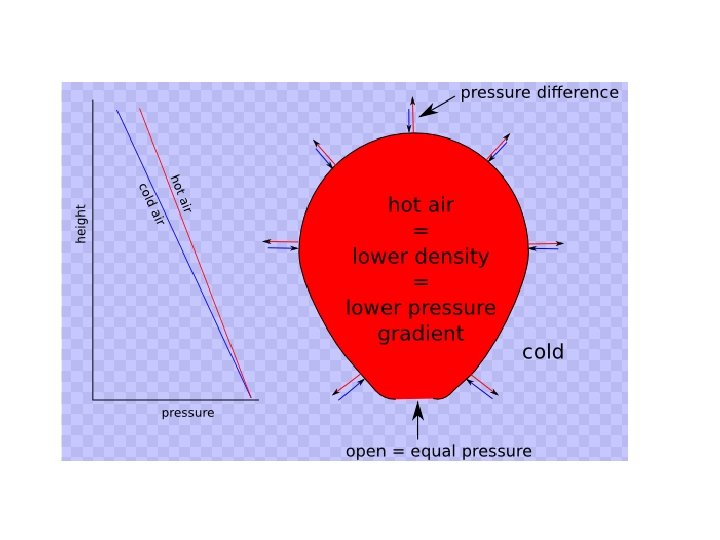
When pressure and amount of gas molecules is held constant: • As temperature goes up volume goes up • As temperature goes down, volume goes down DIRECT RELATIONSHIP

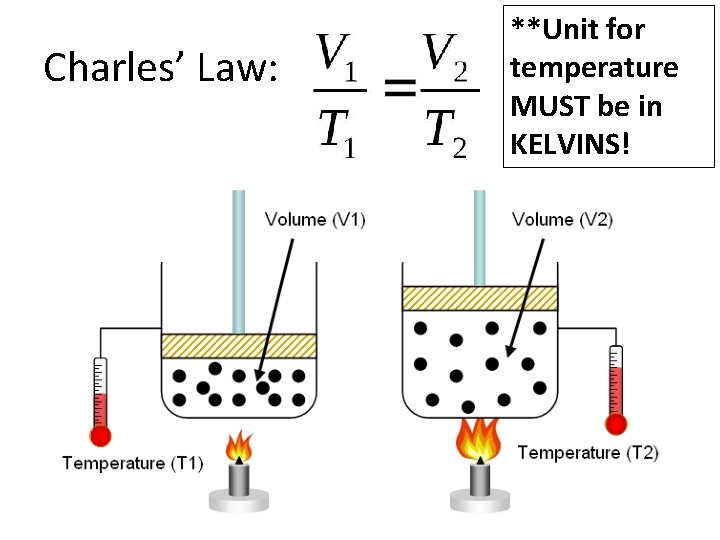
Charles’ Law: **Unit for temperature MUST be in KELVINS!

What about the relationship between temperature and pressure at a constant volume? (Gay-Lussac’s Law) • Temperature must still be in units of Kelvin! • Direct relationship

Problem #1 Temperature will affect airships in Tyria – a fantasy world. The small airships have an initial volume of 4, 200 L. To replicate atmospheric conditions in Tyria scientists place it in a chamber and reduce the temperature from 20 to -60 o. C. What will the final volume of the airship be?

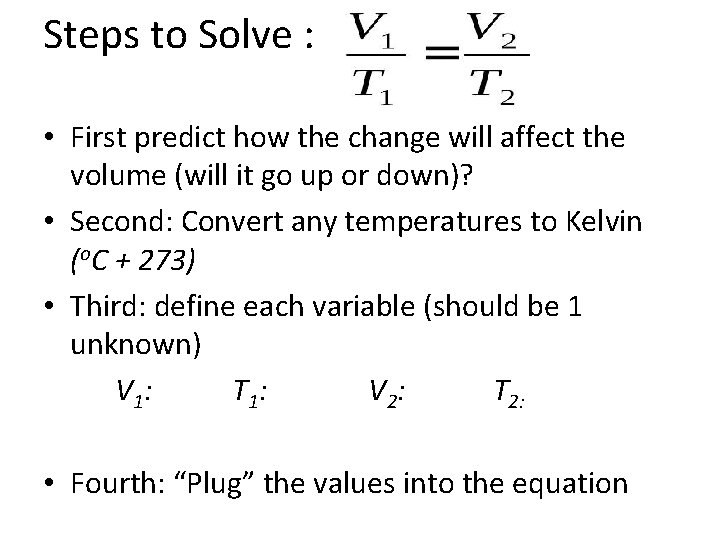
Steps to Solve : • First predict how the change will affect the volume (will it go up or down)? • Second: Convert any temperatures to Kelvin (o. C + 273) • Third: define each variable (should be 1 unknown) V 1 : T 1: V 2: T 2: • Fourth: “Plug” the values into the equation

Problem #1:

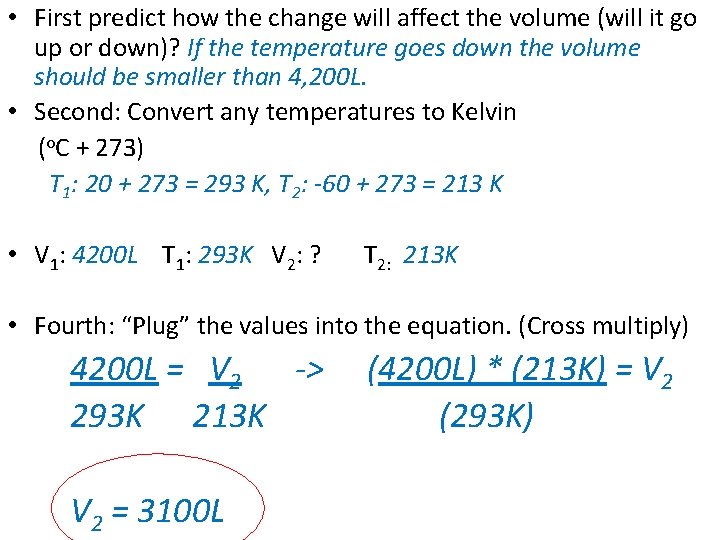
• First predict how the change will affect the volume (will it go up or down)? If the temperature goes down the volume should be smaller than 4, 200 L. • Second: Convert any temperatures to Kelvin (o. C + 273) T 1: 20 + 273 = 293 K, T 2: -60 + 273 = 213 K • V 1: 4200 L T 1: 293 K V 2: ? T 2: 213 K • Fourth: “Plug” the values into the equation. (Cross multiply) 4200 L = V 2 -> 293 K 213 K V 2 = 3100 L (4200 L) * (213 K) = V 2 (293 K)

When problem has been checked off: 1. Complete A. 11 1 -4 AND A. 11 Extra Practice 1 -6 2. Concept Check 1 -3 All are due next class meeting

 The relationship between temperature and volume
The relationship between temperature and volume Relationship between pressure, volume and temperature
Relationship between pressure, volume and temperature Combined gas laws
Combined gas laws Temperature to volume relationship
Temperature to volume relationship Boyles laww
Boyles laww Relationship between temperature and volume
Relationship between temperature and volume Graham's law in real life
Graham's law in real life Newton's first law and second law and third law
Newton's first law and second law and third law Si unit of newton's first law
Si unit of newton's first law Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Relationship between temperature and latitude
Relationship between temperature and latitude Pressure unit
Pressure unit Relationship between heat and temperature
Relationship between heat and temperature