Temperature and Ideal Gases Thermal Equilibrium When two

Temperature and Ideal Gases

Thermal Equilibrium When two substances interact with each othermal energy will be transferred from the hot objet to the cooler object until they both reach the same temperature. This common temperature is called “Thermal Equilibrium”?

Zeroth Law of Thermodynamics If two objects are each in thermal equilibrium with a third object, then those 2 are in thermal equilibrium with each other.

Temperature is proportional to the average KE of within a substance. The rate at which the substance increases temperature is related to the specific heat capacity of the substance. Examples: Water resists change of temperature more than land because of its high specific heat. Metals change temperature easily because of a low specific heat.

Temperature Scales Kelvin Based on Absolute Zero K= o. C + 273. 15 Fahrenheit Based on freezing and boiling point of water with salts added. o. F= 9/5 o. C + 32 Celsius Based on freezing and boiling point of pure water
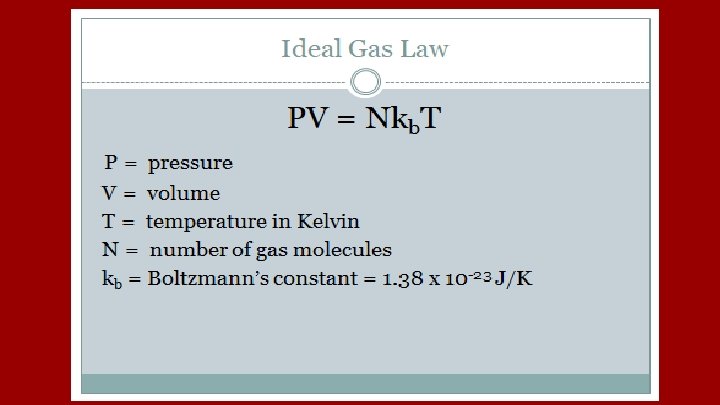

At Constant Temp P 1 V 1 = P 2 V 2 At Constant Pressure V 1/T 1 = V 2/T 2 At Constant Volume P 1/T 1 = P 2/T 2 All math requires the use of the Kelvin Scale for Temperature

Ideal Gases Undergo Perfectly Elastic Collisions The force on the walls of the container can be calculated from the change in momentum of the gas molecules as they collide with the container. This can be used to derive the following relationship PV = NKBT = 1/3 Nmv 2 where v is an average “Root Mean Velocity of all Molecules” Root Mean is found by averaging the squares of the speeds taking the square root of that average.

Internal Energy of a Monatomic Gas Because particles in an ideal gas do not vibrate or rotate the total kinetic energy of all the molecules is equal to the total internal energy of the gas. With “N” number of molecules moving at an average velocity of “v” within a system the total internal energy (U) can be found as… U= N(½m vrms 2)

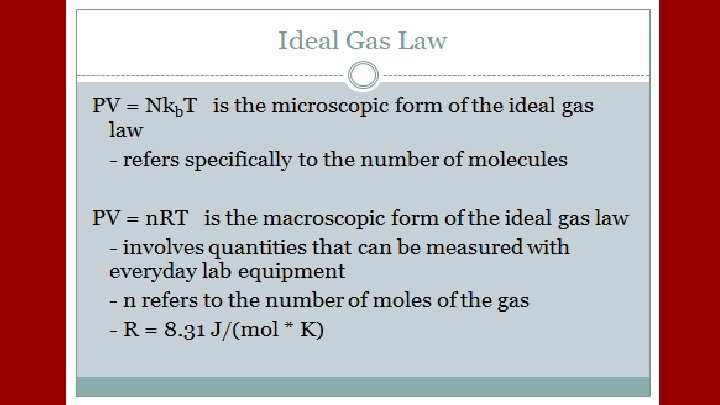
From NKBT = 1/3 Nmv 2, Total Kinetic Energy Can be found to be Kinetic Energy is ½m vrms 2 = 3/2 KBT and Total KE = N 3/2 KBT All the energy in a Monatomic Ideal Gas is Kinetic so U becomes N 3/2 KBT = 3/2 n. RT

How much Kinetic and Total energy will 2 moles of helium have at 20 degrees C?
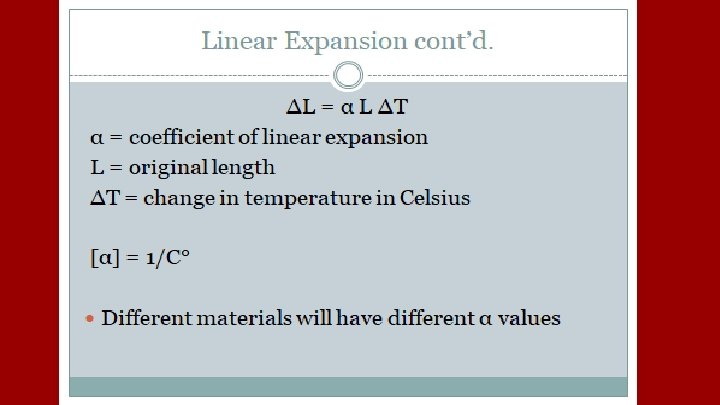
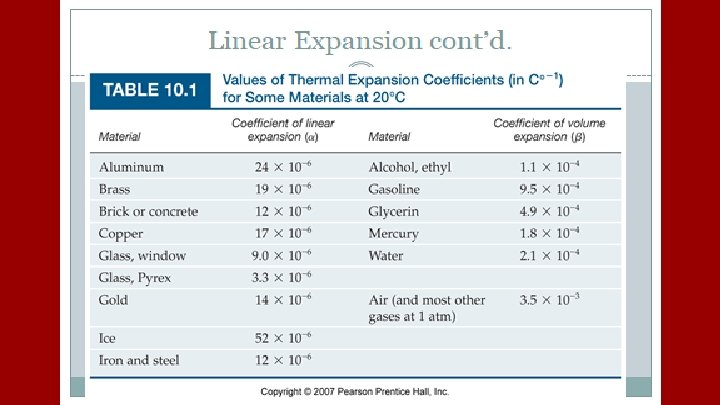

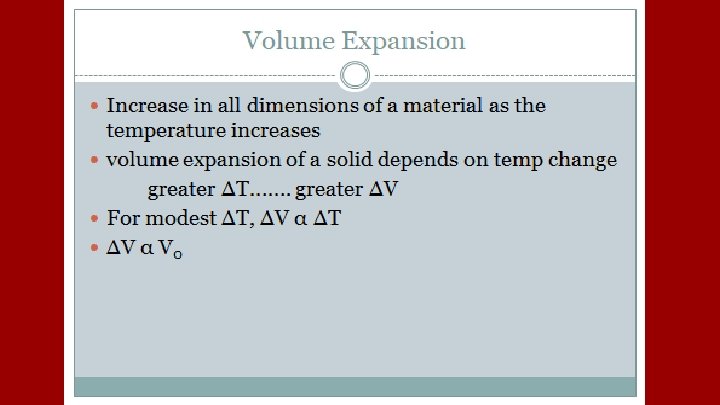

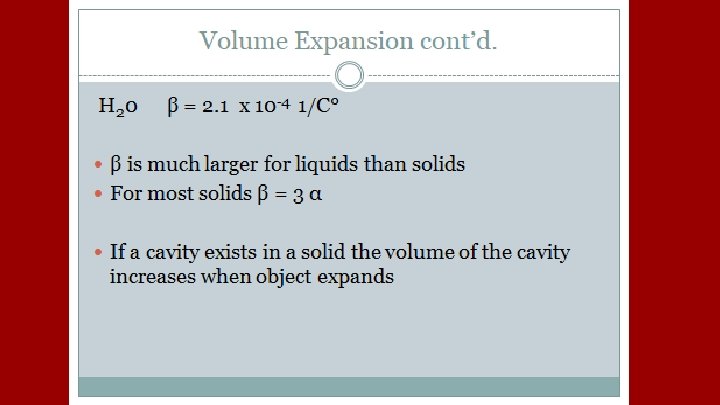
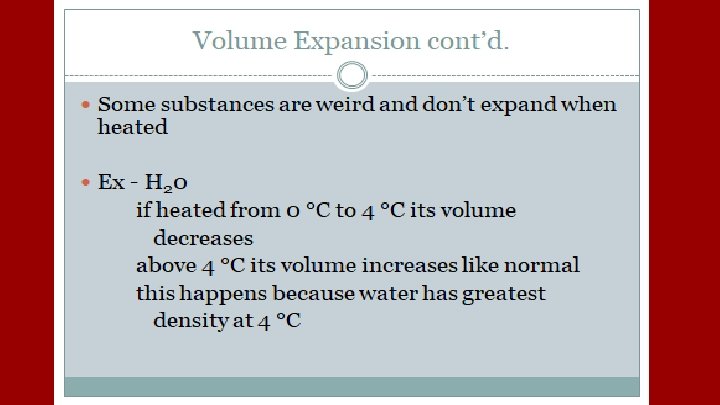

A plastic container serves as a coolant reservoir, catches the radiator fluid (β= 410 x 10 -6/C)that overflows when a car’s engines become hot. The radiator is made of copper (α= 51 x 10 -6/C). If the radiator has a capacity of 15 L and the reservoir is initially empty, how much overlow will it receive when the engine moves from 3 o. C to 92 o. C?

Homework Pgs 361 -363 20, 25, 33, 35, 49, 54, 72, 73 84
- Slides: 22