Temperature and Heat Introduction All matter is composed





















- Slides: 37

Temperature and Heat Introduction

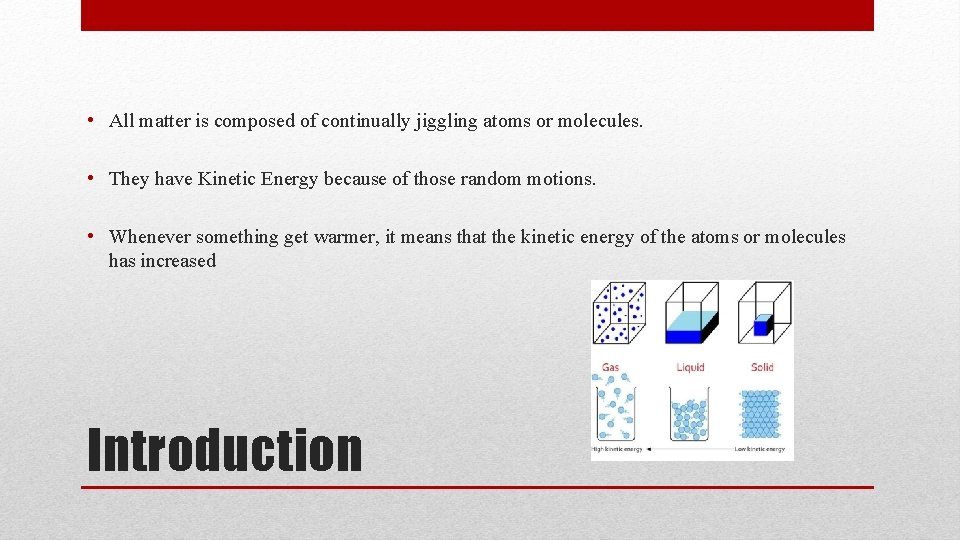
• All matter is composed of continually jiggling atoms or molecules. • They have Kinetic Energy because of those random motions. • Whenever something get warmer, it means that the kinetic energy of the atoms or molecules has increased Introduction

• Temperature is a quantity that tells how hot or cold something is. Compared with the standard. • It also mean a measure of average kinetic energy of the particles in a sample. • Energy that transfers from one object to another because of a temperature different between then is called heat. Temperature & Heat

Thermal physics

• The Celsius scale is based on the properties of water. • Zero Celsius (32 Fahrenheit) is the freezing point of water. • 100 Celsius (212 Fahrenheit) is the boiling point of the water • Degrees is the gap between freezing and boiling is divided into 100 equal parts. Celsius scale

• Fahrenheit scale is the temperature scale used commonly in the United States • Water freezes at 32 • Water boils at 212 Fahrenheit scale

• SI unit for temperature • Degree are the same size as the Celsius degree are called Kelvin. • At Absolute zero a substance has no kinetic energy to give up. Kelvin scale

• Conversion Formulas

• 1. Convert 65 Degrees Celsius to Fahrenheit and Kelvin • 2. Convert 444 K to Degrees Celsius and Fahrenheit Example

Temperature and kinetic energy

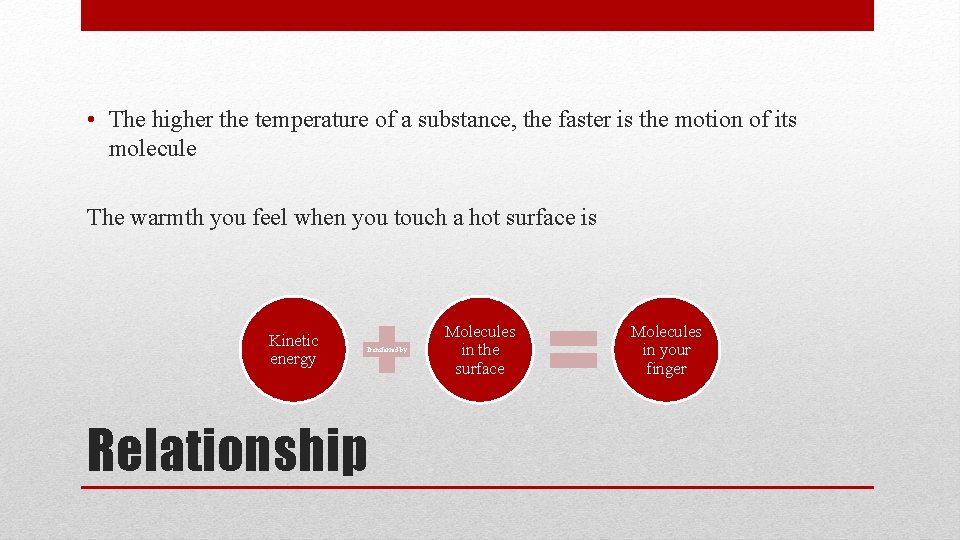
• The higher the temperature of a substance, the faster is the motion of its molecule Relationship

• The higher the temperature of a substance, the faster is the motion of its molecule The warmth you feel when you touch a hot surface is Kinetic energy Transferred by Relationship Molecules in the surface Molecules in your finger

Heat, Thermal equilibrium, and internal energy

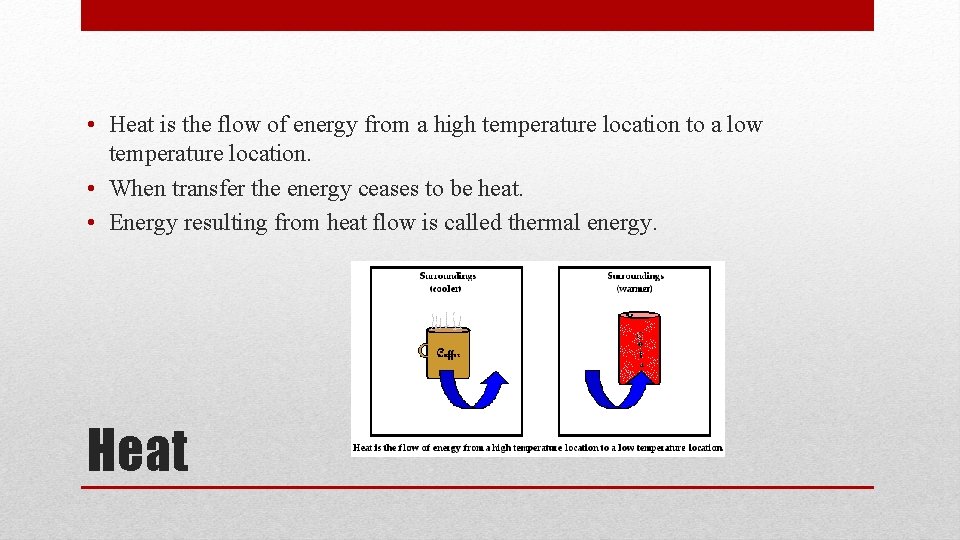
• Heat is the flow of energy from a high temperature location to a low temperature location. • When transfer the energy ceases to be heat. • Energy resulting from heat flow is called thermal energy. Heat

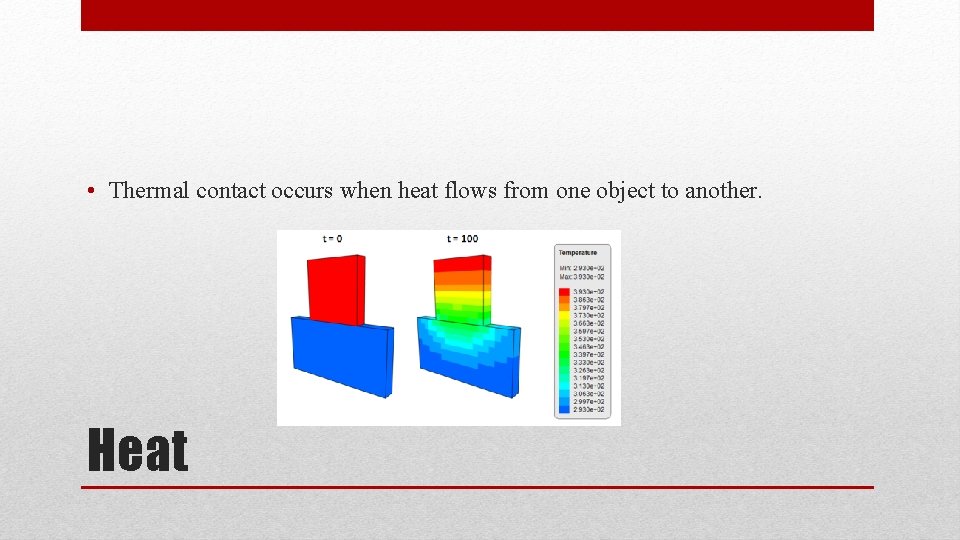
• Thermal contact occurs when heat flows from one object to another. Heat

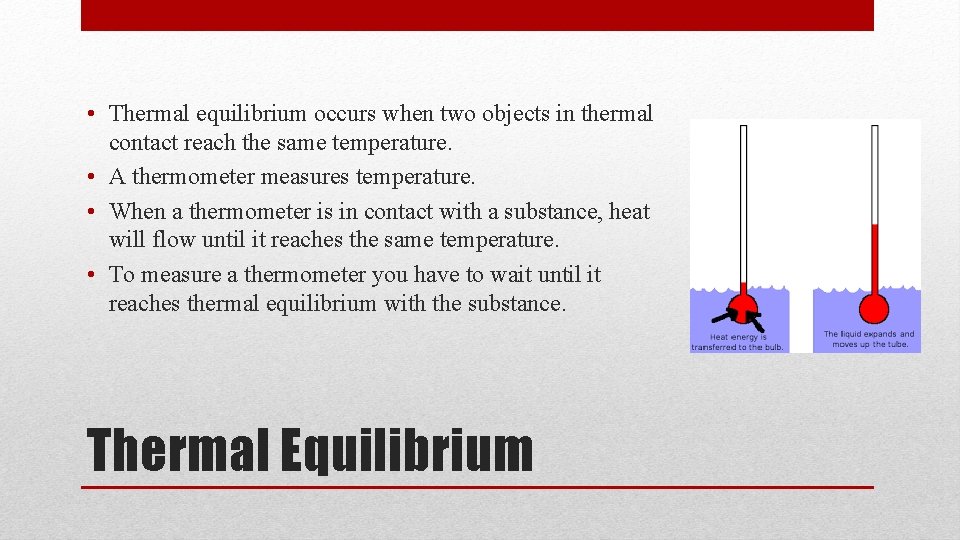
• Thermal equilibrium occurs when two objects in thermal contact reach the same temperature. • A thermometer measures temperature. • When a thermometer is in contact with a substance, heat will flow until it reaches the same temperature. • To measure a thermometer you have to wait until it reaches thermal equilibrium with the substance. Thermal Equilibrium

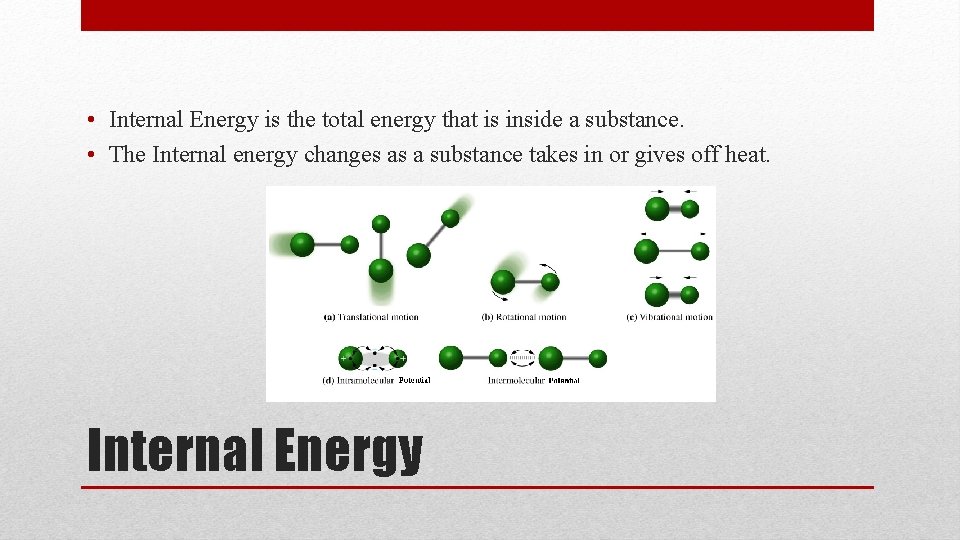
• Internal Energy is the total energy that is inside a substance. • The Internal energy changes as a substance takes in or gives off heat. Internal Energy

• Internal Energies: • • Kinetic energy of colliding molecules Rotational kinetic energy of molecules Kinetic energy due to movements of atoms within molecules Potential energy due to forces between molecules Internal Energy

Measurements of heat

• To determine the amount of heat transferred, measure the temperature change of a known mass of a substance that absorbs heat. • When heat is transferred from an object to the other, there is a change in temperature. • Heat can be calculated by identifying the mass and type of material • Even though the same amount of heat is added, the temperature in a container with less water increases more.

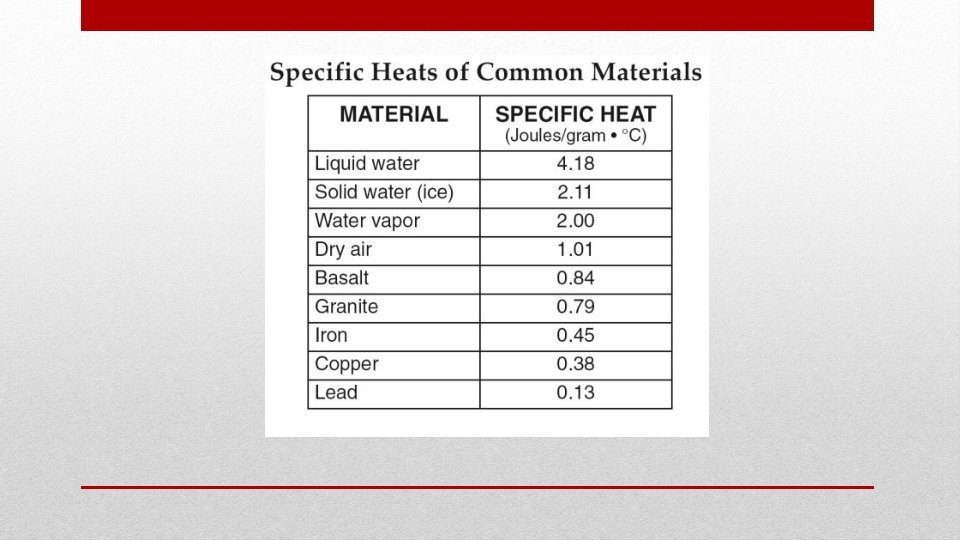
• Heat is commonly expressed in either of two units: • calorie, an older metric unit • Defined as the amount of heat required to raise the temperature of 1 gram of water by 1 Celsius an English unit commonly used in the United States • Scientists express heat in terms of the Joule , an SI unit used or all forms of energy. • British thermal unit (Btu), • 1 calorie = 4. 186 J • 1 Kilocalorie = 1000 calories Units of Heat

Heat Capacity

• Heat Capacity- is the heat required to raise the temperature of an object by 1 degree • Specific heat capacity- quantity of heat required to raise the temperature of 1 gram by 1 degree Heat Capacity



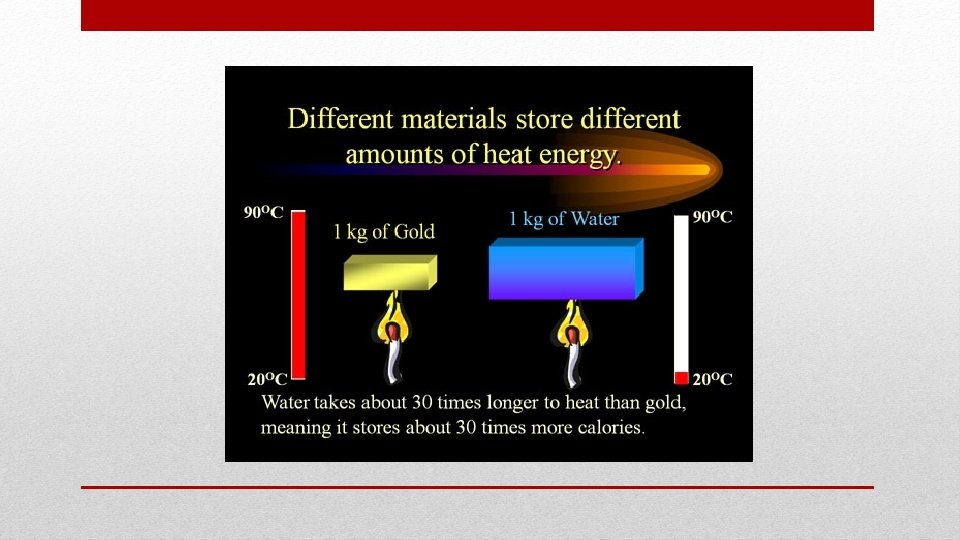
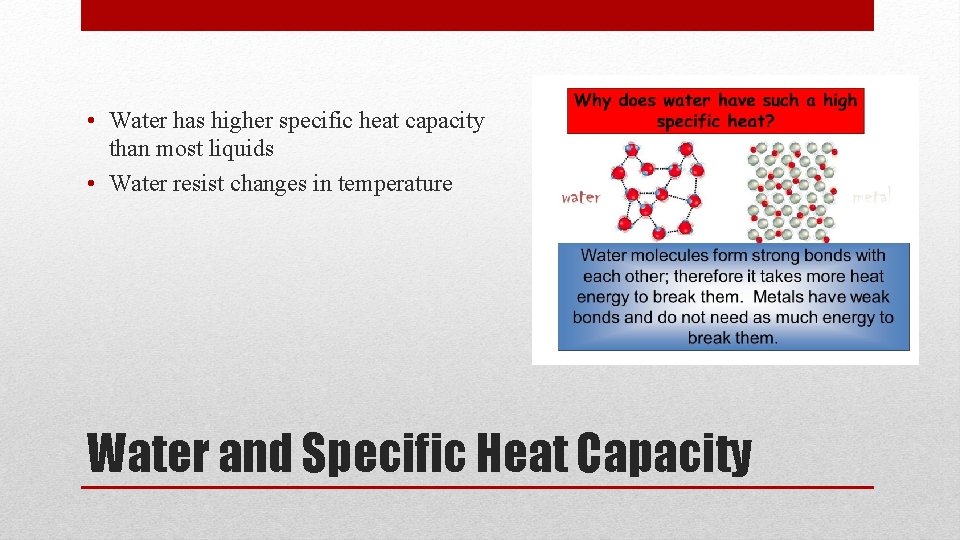
• Water has higher specific heat capacity than most liquids • Water resist changes in temperature Water and Specific Heat Capacity

Thermal Expansion and Application

• Most forms of matter expand when they are heated and contract when they are cool. Thermal Expansion

• Expansion Joint is a gap between material that expands and contracts on varying temperature. Application: Expansion Joints

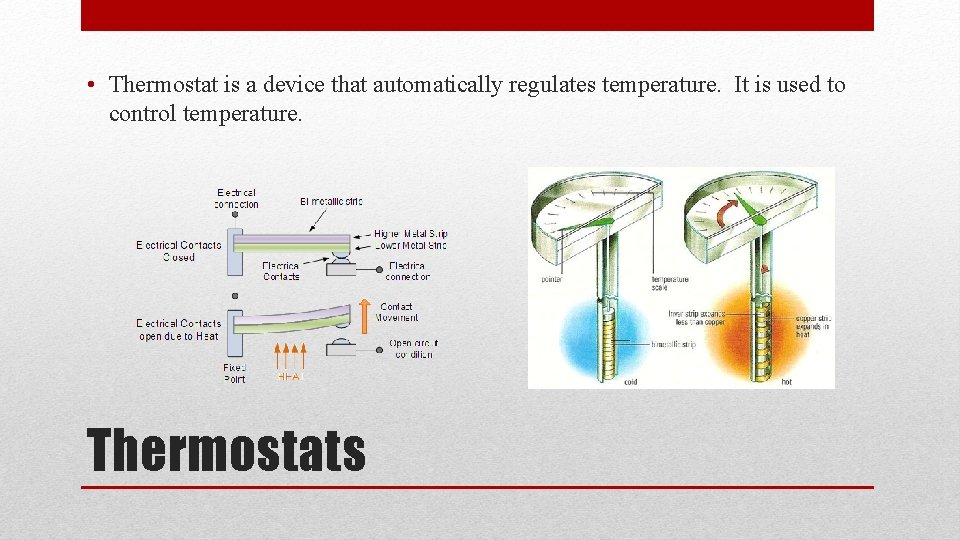
• In a bimetallic strip, two strips of different metals are welded or riveted together. • The movement of the strip can turn a pointer , regulate a valve, or operate a switch. Application: Bimetallic Strips

• Thermostat is a device that automatically regulates temperature. It is used to control temperature. Thermostats

• If one part of a piece of glass is heated or cooled more rapidly than the adjacent parts, the expansion or contraction may break the glass. Glasses

Thermal Expansion and Application

APPLICATIONS

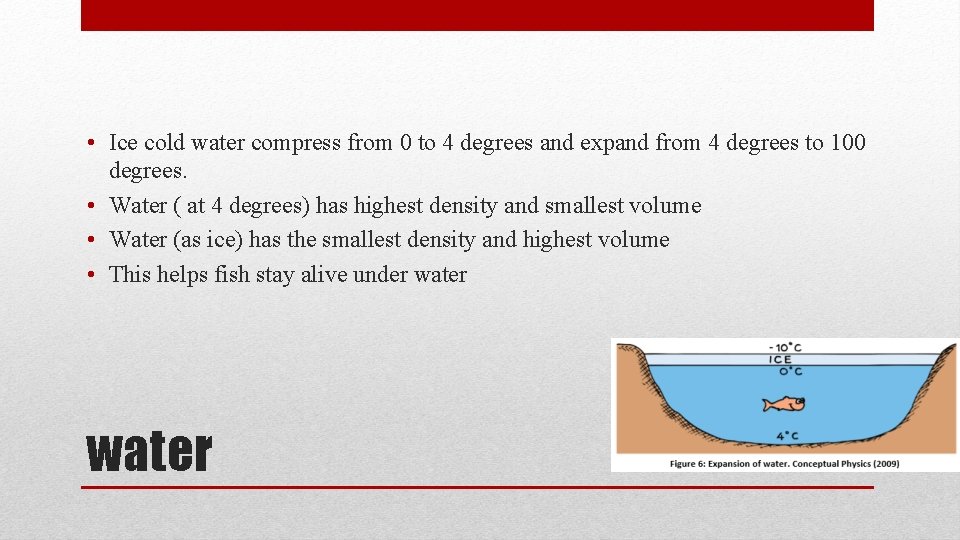
• Ice cold water compress from 0 to 4 degrees and expand from 4 degrees to 100 degrees. • Water ( at 4 degrees) has highest density and smallest volume • Water (as ice) has the smallest density and highest volume • This helps fish stay alive under water

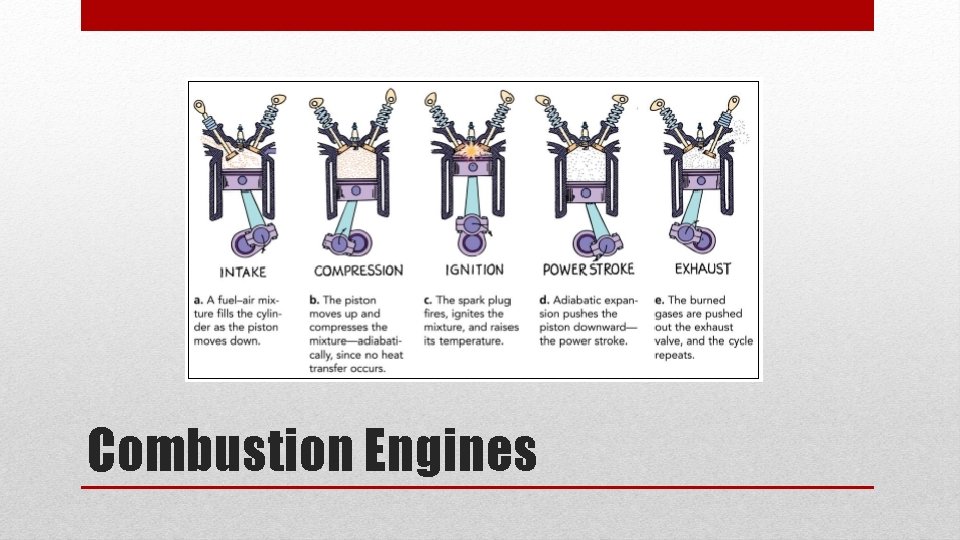
Combustion Engines

• Mercury expands as temperature increases Thermometer