Technology Appraisal of Medical Devices at NICE Methods








- Slides: 23

Technology Appraisal of Medical Devices at NICE – Methods and Practice Mark Sculpher Professor of Health Economics Centre for Health Economics University of York, UK

Outline • • • Policy context of NICE methods Are devices different? The role of randomised trials When do we have sufficient evidence? Impact of NICE guidance

Background • Brief overview of NICE • NICE’s decisions on devices • NICE’s methods: requirement for decision making • Are devices different? • The impact of NICE guidance

The National Institute for Health and Clinical Excellence (NICE) • Following election of Labour government 1997 • Prolonged controversy about ‘post code prescribing’ in the UK National Health Service • Wish to ‘de-politicize’ decisions about which technologies to cover in NHS • Desire to use best available methods to address difficult questions • Focus on drugs but devices also included

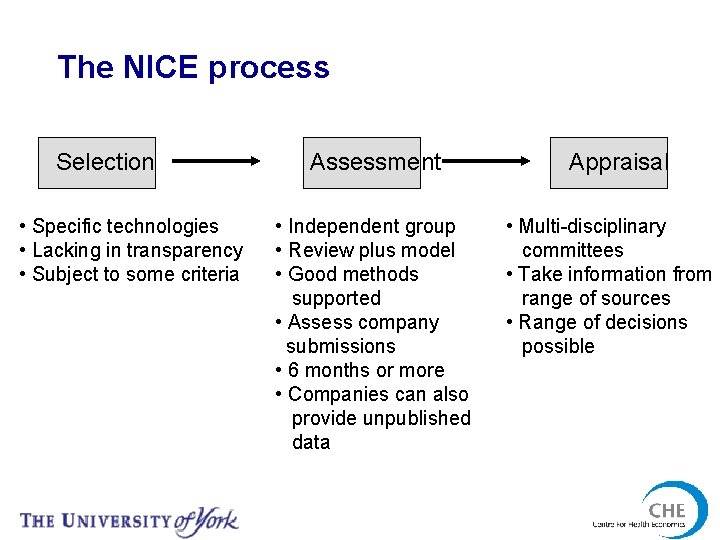
The NICE process Selection • Specific technologies • Lacking in transparency • Subject to some criteria Assessment • Independent group • Review plus model • Good methods supported • Assess company submissions • 6 months or more • Companies can also provide unpublished data Appraisal • Multi-disciplinary committees • Take information from range of sources • Range of decisions possible

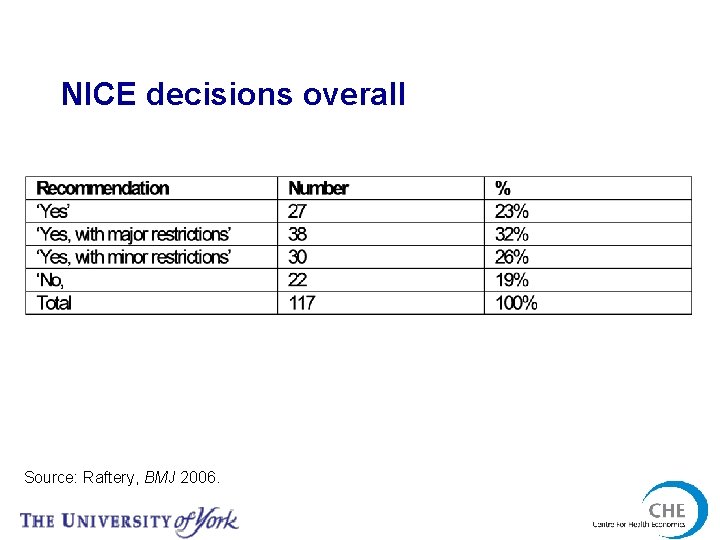
NICE decisions overall Source: Raftery, BMJ 2006.

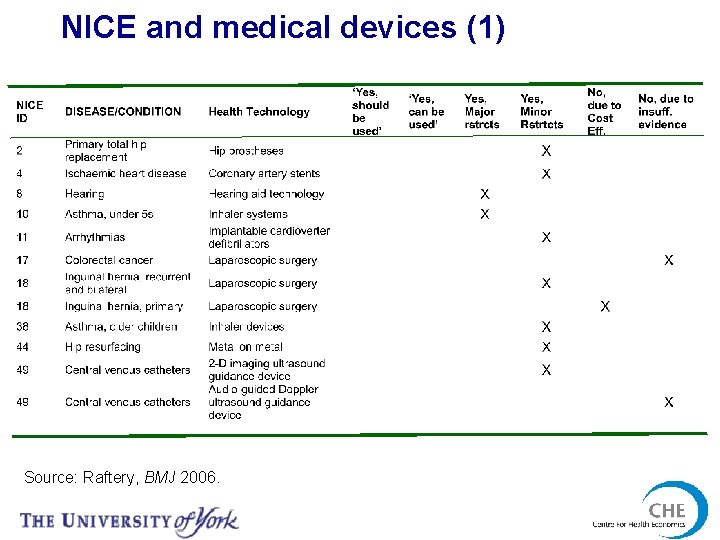
NICE and medical devices (1) Source: Raftery, BMJ 2006.

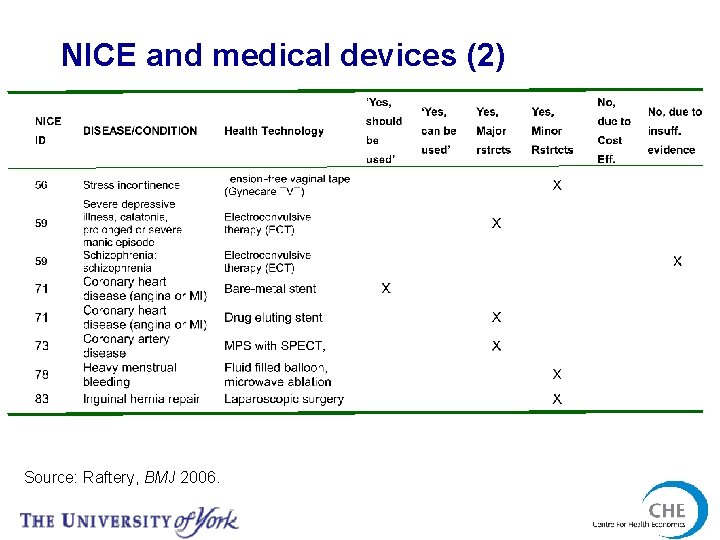
NICE and medical devices (2) Source: Raftery, BMJ 2006.

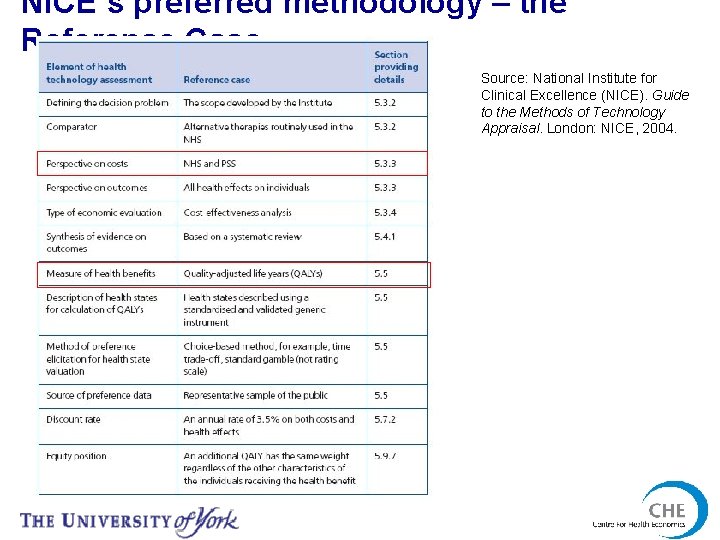
NICE and methods • 2004 guidance more prescriptive than most international guidelines • Based on a clear view about the use of economic evaluation for decision making • Included the concept of the Reference Case National Institute for Clinical Excellence (NICE). Guide to the Methods of Technology Appraisal. London: NICE, 2004.

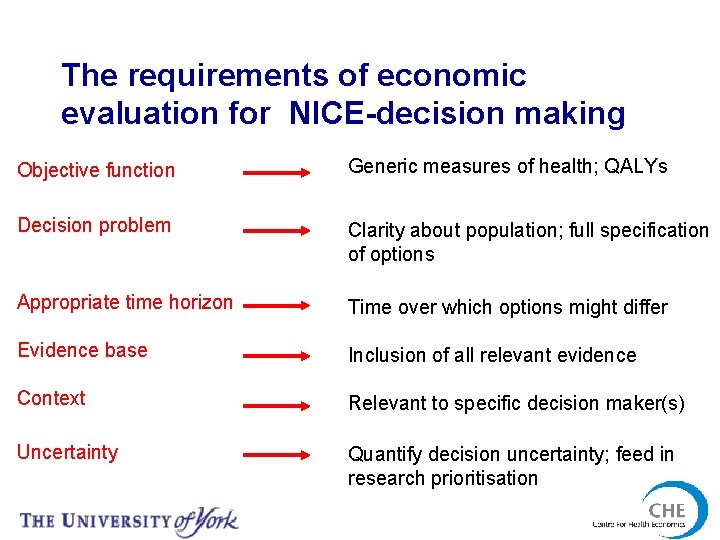
The requirements of economic evaluation for NICE-decision making Objective function Generic measures of health; QALYs Decision problem Clarity about population; full specification of options Appropriate time horizon Time over which options might differ Evidence base Inclusion of all relevant evidence Context Relevant to specific decision maker(s) Uncertainty Quantify decision uncertainty; feed in research prioritisation

NICE’s preferred methodology – the Reference Case Source: National Institute for Clinical Excellence (NICE). Guide to the Methods of Technology Appraisal. London: NICE, 2004.

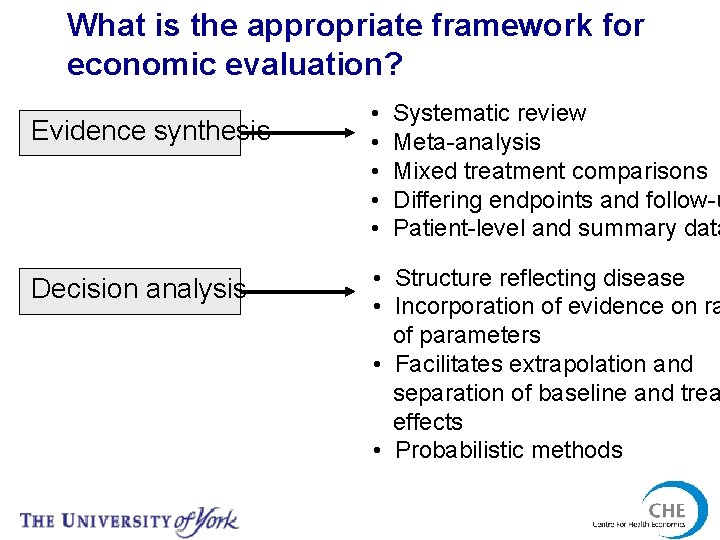
What is the appropriate framework for economic evaluation? Evidence synthesis Decision analysis • • • Systematic review Meta-analysis Mixed treatment comparisons Differing endpoints and follow-u Patient-level and summary data • Structure reflecting disease • Incorporation of evidence on ra of parameters • Facilitates extrapolation and separation of baseline and trea effects • Probabilistic methods

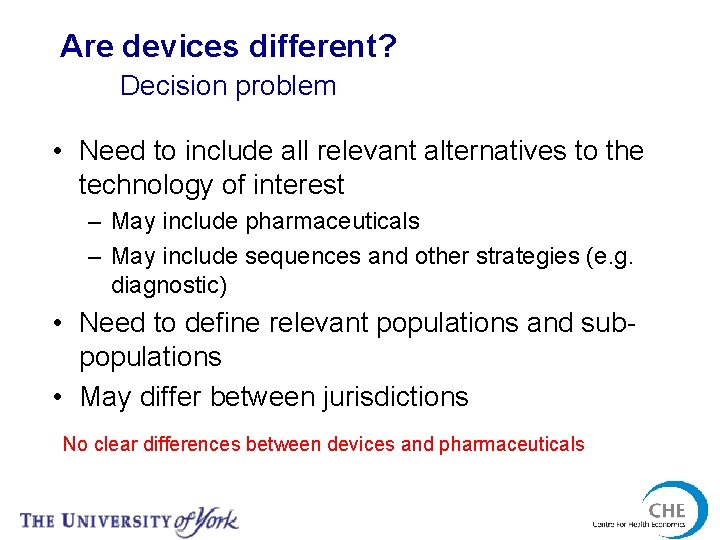
Are devices different? Decision problem • Need to include all relevant alternatives to the technology of interest – May include pharmaceuticals – May include sequences and other strategies (e. g. diagnostic) • Need to define relevant populations and subpopulations • May differ between jurisdictions No clear differences between devices and pharmaceuticals

Are devices different? Evidence base • Less likely to need trials for regulatory purposes • Does not mean should not be used for reimbursement • Typical ‘regulatory’ trials have limitations for economic evaluation • The evolution of devices over time – Not unique to devices – Has implications for evidence gathering – Need larger longitudinal studies, sub-groups on device types – Comparators may also be changing over time

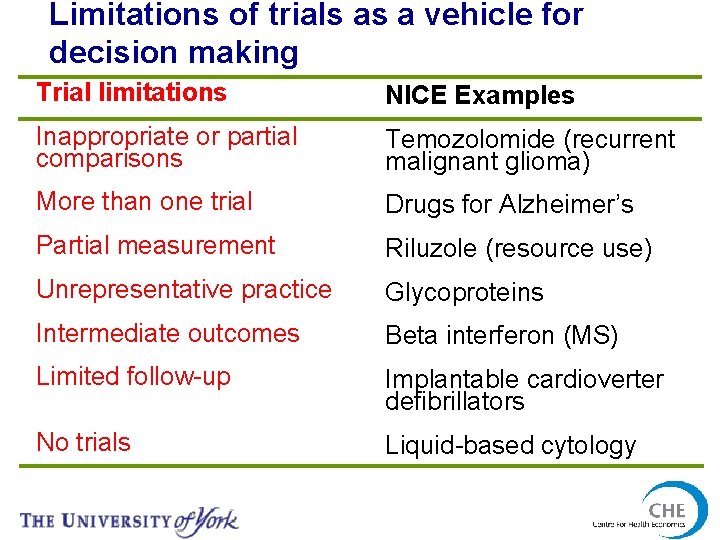
Limitations of trials as a vehicle for decision making Trial limitations NICE Examples Inappropriate or partial comparisons Temozolomide (recurrent malignant glioma) More than one trial Drugs for Alzheimer’s Partial measurement Riluzole (resource use) Unrepresentative practice Glycoproteins Intermediate outcomes Beta interferon (MS) Limited follow-up Implantable cardioverter defibrillators No trials Liquid-based cytology

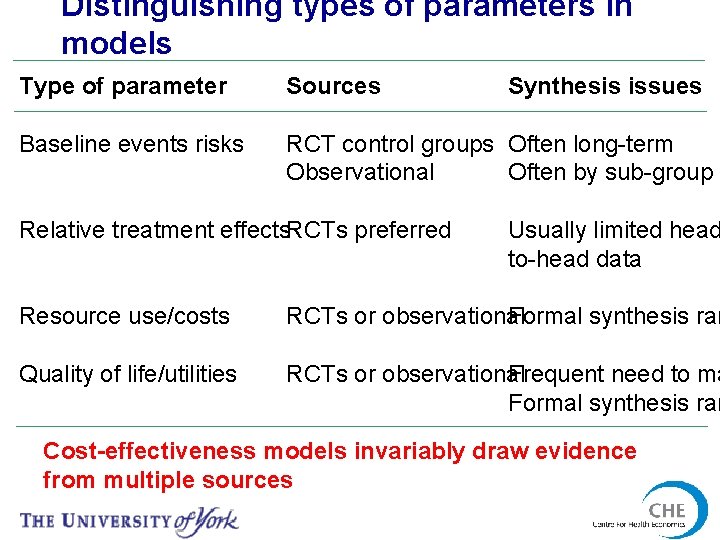
Distinguishing types of parameters in models Type of parameter Sources Baseline events risks RCT control groups Often long-term Often by sub-group Observational Relative treatment effects. RCTs preferred Synthesis issues Usually limited head to-head data Resource use/costs Formal synthesis rar RCTs or observational Quality of life/utilities Frequent need to ma RCTs or observational Formal synthesis rar Cost-effectiveness models invariably draw evidence from multiple sources

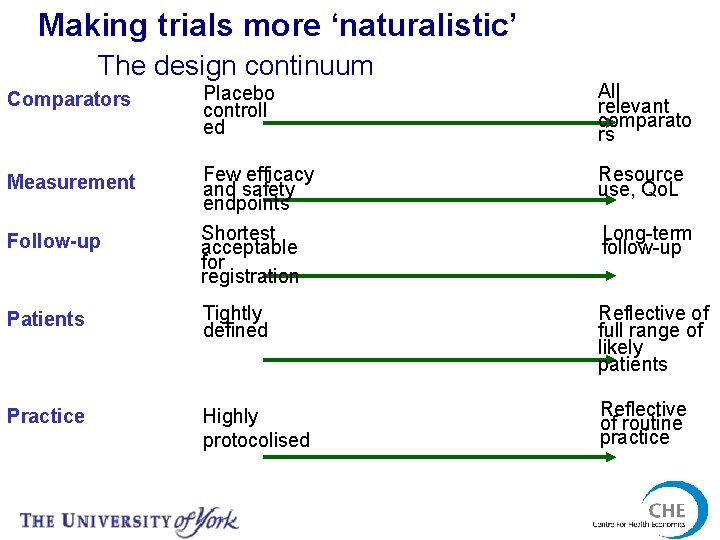
Making trials more ‘naturalistic’ The design continuum Comparators Placebo controll ed All relevant comparato rs Measurement Few efficacy and safety endpoints Shortest acceptable for registration Resource use, Qo. L Patients Tightly defined Reflective of full range of likely patients Practice Highly protocolised Reflective of routine practice Follow-up Long-term follow-up

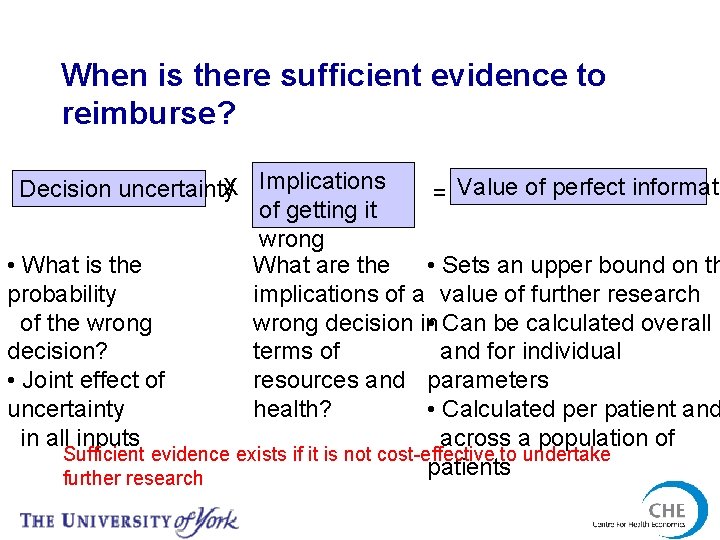
When is there sufficient evidence to reimburse? Decision uncertainty. X Implications = Value of perfect informati of getting it wrong • What is the What are the • Sets an upper bound on th probability implications of a value of further research of the wrong decision in • Can be calculated overall decision? terms of and for individual • Joint effect of resources and parameters uncertainty health? • Calculated per patient and in all inputs across a population of Sufficient evidence exists if it is not cost-effective to undertake patients further research

Evidence on impact of NICE decision on the NHS

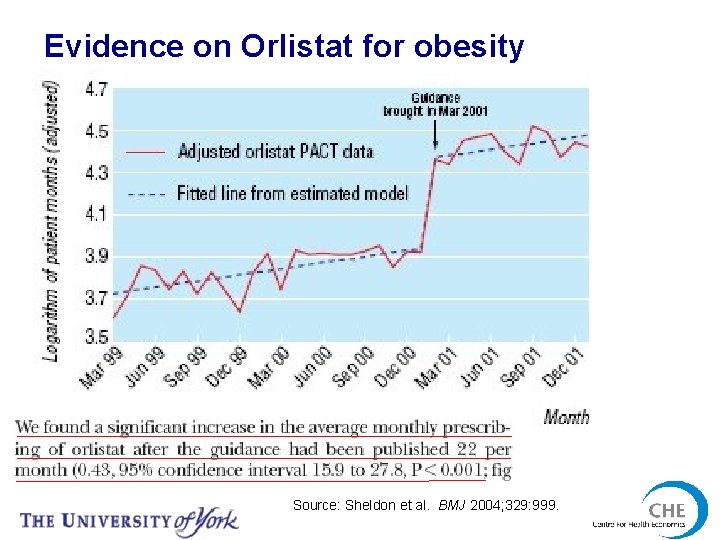
Evidence on Orlistat for obesity Source: Sheldon et al. BMJ 2004; 329: 999.

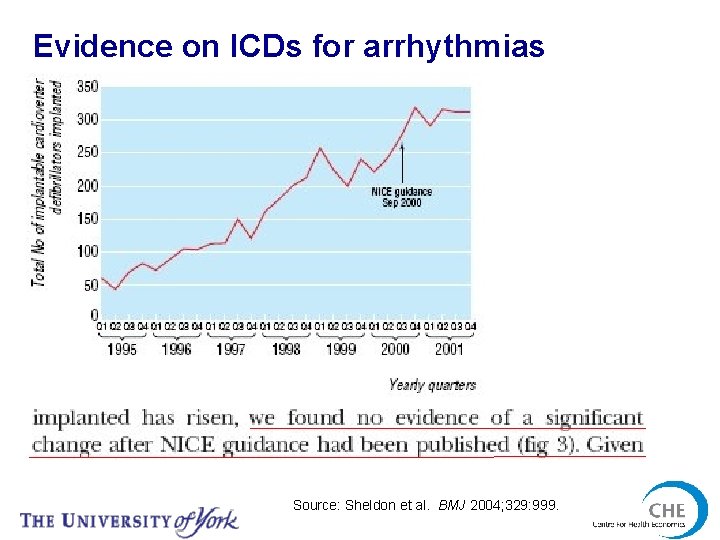
Evidence on ICDs for arrhythmias Source: Sheldon et al. BMJ 2004; 329: 999.

What influences uptake? Source: Sheldon et al. BMJ 2004; 329: 999.

Conclusions • NICE part of an international trend towards greater use of economics in decision making • NICE is prescriptive about methods • Few differences between drugs and devices which affect appropriate methods • Impact of NICE guidance has been variable