Tecfidera Use in Multiple Sclerosis A Patient Case










![Tecfidera® Adverse Reactions Tecfidera® [package insert]. Cambridge, MA: Biogen, Inc; Tecfidera® Adverse Reactions Tecfidera® [package insert]. Cambridge, MA: Biogen, Inc;](https://slidetodoc.com/presentation_image/50ef065a2da9a42f05309316809a5fd8/image-22.jpg)






- Slides: 29

Tecfidera® Use in Multiple Sclerosis: A Patient Case Stefanie L. Drahuschak Pharm. D Candidate Class of 2014 University of Pittsburgh School of Pharmacy Walgreens Specialty Pharmacy March 12, 2014

Objectives � 1) Review the basics of Multiple Sclerosis as a disease state. � 2) Understand the important counseling points, adverse events, and dosing of Tecfidera®. � 3) Apply the knowledge of MS and Tecfidera® to a specific patient case.

Patient: CH � 57 yo female �No known drug allergies �Diagnosis: Multiple Sclerosis ◦ ICD 9 Code: 340

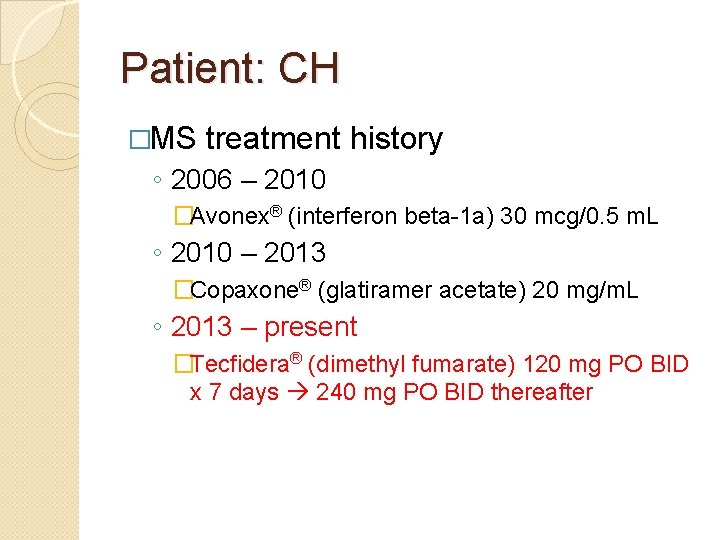
Patient: CH �MS treatment history ◦ 2006 – 2010 �Avonex® (interferon beta-1 a) 30 mcg/0. 5 m. L ◦ 2010 – 2013 �Copaxone® (glatiramer acetate) 20 mg/m. L ◦ 2013 – present �Tecfidera® (dimethyl fumarate) 120 mg PO BID x 7 days 240 mg PO BID thereafter

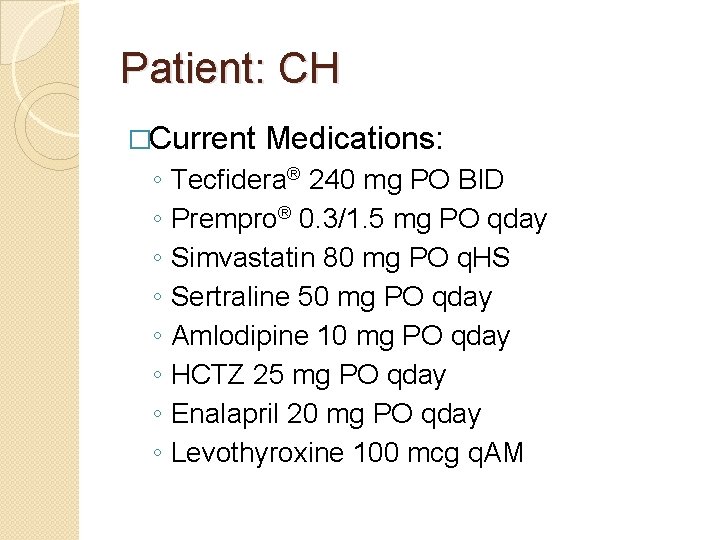
Patient: CH �Current ◦ ◦ ◦ ◦ Medications: Tecfidera® 240 mg PO BID Prempro® 0. 3/1. 5 mg PO qday Simvastatin 80 mg PO q. HS Sertraline 50 mg PO qday Amlodipine 10 mg PO qday HCTZ 25 mg PO qday Enalapril 20 mg PO qday Levothyroxine 100 mcg q. AM

Multiple Sclerosis �MS is an inflammatory disease of the CNS �The term multiple sclerosis refers to two characteristics of the disease: ◦ 1) Multiple areas of brain and spinal cord affected producing multiple neurologic symptoms ◦ 2) Characteristic plaques or sclerosed areas Bainbridge JL, Corboy JR. “Multiple Sclerosis. ” Pharmacotherapy, Ed. Dipiro JT, Talbert RL, Yee GC, et al. New York: Mc. Graw-Hill Companies, Inc, 2008.

Epidemiology �Usually diagnosed between 15 -45 yo �~10, 000 new cases diagnosed/yr in US �Woman > men in 2: 1 ratio �Prevalence is higher the greater the distance from equator ◦ Inverse relationship between MS and vitamin D exposure? �Occurs more frequently in whites of Bainbridge JL, Corboy JR. “Multiple Sclerosis. ” Pharmacotherapy, Ed. Dipiro Scandinavian ancestry JT, Talbert RL, Yee GC, et al. New York: Mc. Graw-Hill Companies, Inc, 2008.

Clinical Presentation �Primary • Ataxia • Speech complaints/optic difficulty neuritis • Psychological • Gait problems and changes falls • Cognitive • Parasthesias changes • Pain • Fatigue • Spasticity • Bowel/bladder • Weakness dysfunction • Sexual dysfunction • Tremor. Ed. Dipiro JT, Bainbridge JL, Corboy JR. “Multiple Sclerosis. ” Pharmacotherapy, S/Sx • Visual Talbert RL, Yee GC, et al. New York: Mc. Graw-Hill Companies, Inc, 2008. 963 -978.

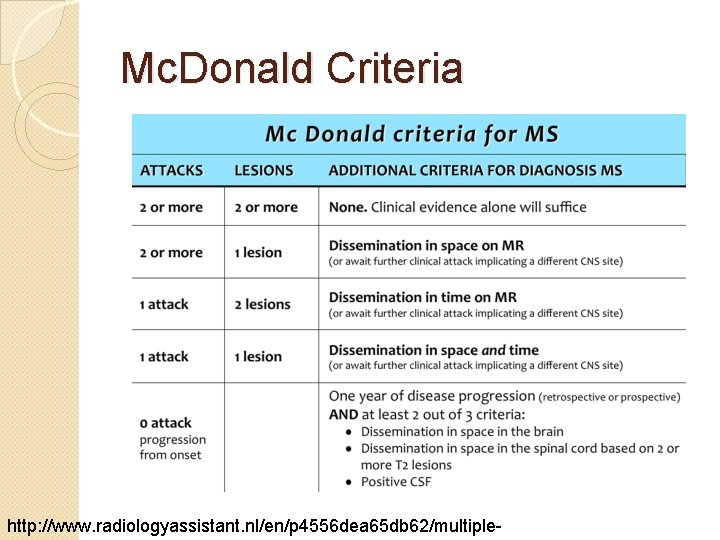
Diagnosis �Diagnosis of exclusion ◦ Sx can often be attributed to other neurological issues �Occurrence of at least 2 episodes of neurologic disturbance reflecting distinct sites of damage in the CNS that cannot be explained by another mechanism �Since 2010, Mc. Donald criteria has been considered the gold standard for diagnosis Bainbridge JL, Corboy JR. “Multiple Sclerosis. ” Pharmacotherapy, Ed. Dipiro JT, Talbert RL, Yee GC, et al. New York: Mc. Graw-Hill Companies, Inc, 2008.

Mc. Donald Criteria http: //www. radiologyassistant. nl/en/p 4556 dea 65 db 62/multiple-

Types of MS � 1) Relapsing-Remitting MS (RRMS) ◦ Most common, about 85% initially diagnosed � 2) Secondary-Progressive MS (SPMS) ◦ Symptoms worsen steadily over time � 3) Primary-Progressive MS (PPMS) ◦ Only ~10% of patients � 4) Progressive-Relapsing MS (PRMS) ◦ Rare (5%) Bainbridge JL, Corboy JR. “Multiple Sclerosis. ” Pharmacotherapy, Ed. Dipiro JT, Talbert RL, Yee GC, et al. New York: Mc. Graw-Hill Companies,

Treatment �Three broad categories ◦ 1) Symptomatic therapy ◦ 2) Treatment of acute attacks ◦ 3) Disease-modifying therapy

Symptomatic Therapy Tx �Gait problems ◦ Ampyra® (dalfampridine) �Fatigue ◦ Modafinil (Provigil®) �Depression ◦ SSRIs �Sexual dysfunction ◦ Cialis®, Viagra® Bainbridge JL, Corboy JR. “Multiple Sclerosis. ” Pharmacotherapy, Ed. Dipiro JT, Talbert RL, Yee GC, et al. New York: Mc. Graw-Hill Companies,

Tx of Acute Attacks �When functional ability is affected, IV high-dose corticosteroids are used ◦ IV methylprednisolone �MOA of corticosteroids is unknown, but suspected that steroids improve recovery by decreasing edema is the area of demyelination Bainbridge JL, Corboy JR. “Multiple Sclerosis. ” Pharmacotherapy, Ed. Dipiro JT, Talbert RL, Yee GC, et al. New York: Mc. Graw-Hill Companies, Inc, 2008.

Disease-Modifying Therapy �Interferons ◦ Betaseron® (IFN-beta 1 b) ◦ Avonex® (IFN-beta 1 a) ◦ Rebif® (IFN-beta 1 a) �Copaxone® (glatiramer acetate) �Tysabri® (natalizumab) �Gilenya® (Fingolimod) �Novantrone® (mitoxantrone) �Tecfidera® (dimethyl fumarate) Bainbridge JL, Corboy JR. “Multiple Sclerosis. ” Pharmacotherapy, Ed. Dipiro JT, Talbert RL, Yee GC, et al. New York: Mc. Graw-Hill Companies, Inc, 2008.

Tecfidera® �Dimethyl fumarate �Indication ◦ Tecfidera® is indicated for the treatment of patients with relapsing forms of multiple sclerosis Tecfidera® [package insert]. Cambridge, MA: Biogen, Inc; 2013.

Mechanism of Action �Mostly unknown �DMF and MMF activate the Nuclear factor (erythroid-derived 2)-like 2 (NRF 2) pathway �NRF 2 pathway is involved in cellular response to oxidative stress Tecfidera® [package insert]. Cambridge, MA: Biogen, Inc;

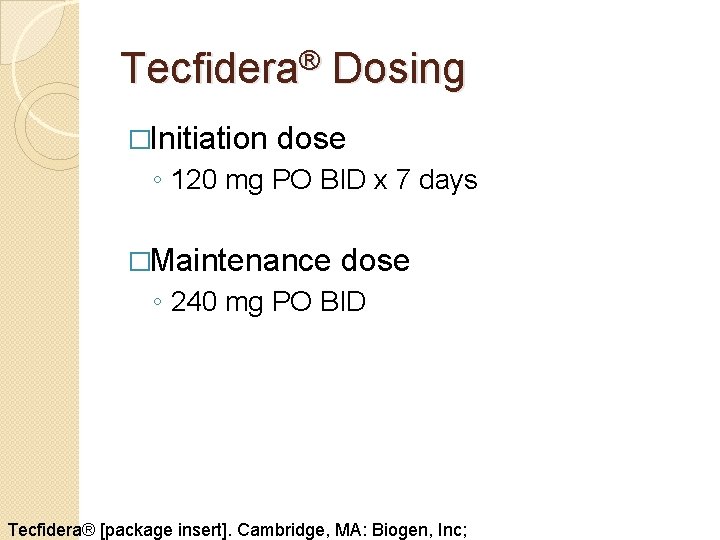
Tecfidera® Dosing �Initiation dose ◦ 120 mg PO BID x 7 days �Maintenance dose ◦ 240 mg PO BID Tecfidera® [package insert]. Cambridge, MA: Biogen, Inc;

Tecfidera® Availability �Hard, gelatin delayed release capsules of 120 or 240 mg ◦ 120 mg: “BG-12 120 mg” on capsule ◦ 240 mg: “BG-12 240 mg” on capsule � 30 -day Starter Pack � 7 -day bottle of 120 mg capsules � 30 -day bottle of 240 mg capsules Tecfidera® [package insert]. Cambridge, MA: Biogen, Inc;

Tecfidera® http: //www. empr. com/tecfidera-approved-a-first-line-oral-multiplesclerosis-treatment/article/286349/

Tecfidera® Administration �Should be swallowed whole and intact �Should NOT be crushed or chewed �Capsule contents should NOT be sprinkled on food �Can be without regard to food �Administration with food may reduce the incidence of flushing Tecfidera® [package insert]. Cambridge, MA: Biogen, Inc;
![Tecfidera Adverse Reactions Tecfidera package insert Cambridge MA Biogen Inc Tecfidera® Adverse Reactions Tecfidera® [package insert]. Cambridge, MA: Biogen, Inc;](https://slidetodoc.com/presentation_image/50ef065a2da9a42f05309316809a5fd8/image-22.jpg)
Tecfidera® Adverse Reactions Tecfidera® [package insert]. Cambridge, MA: Biogen, Inc;

Serious AE - Flushing �Warmth, redness, itching, and/or burning sensation �In clinical trials, 40% experienced �Flushing generally began soon after initiation and improved over time �Initiation dose is used to desensitize body to flushing �Taking medication with food or premedicating with aspirin may help reduce incidence Tecfidera® [package insert]. Cambridge, MA: Biogen,

Serious AE: Lymphopenia �Mean lymphocyte counts decreased by ~30% during the first year of treatment and then remained stable � 4 weeks after d/c, lymphocyte counts increased but did not return to baseline �Before tx, a baseline CBC should be conducted, then annually thereafter Tecfidera® [package insert]. Cambridge, MA: Biogen, Inc;

Pharmacokinetics �Metabolism ◦ Extensively metabolized by esterases ◦ No involvement from CYP 450 system �Elimination ◦ Exhalation of CO 2 is primary route – 60% ◦ Renal and fecal elimination are minor routes, accounting for 16% and 1%, respectively Tecfidera® [package insert]. Cambridge, MA: Biogen, Inc;

Use in Pregnancy �Pregnancy Category C �In animal studies, adverse effects on offspring survival, growth, sexual maturation, and neurobehavioral function were observed at clinically relevant doses �Should only be used if benefit > risk Tecfidera® [package insert]. Cambridge, MA: Biogen, Inc;

Important Counseling Points �Will be provided two strengths when starting therapy ◦ Do not crush, swallow whole �Flushing and GI upset are most common reactions, especially initially ◦ Take with food, aspirin if needed �Inform MD if you become pregnant �Regular blood tests are important to monitor lymphocytes Tecfidera® [package insert]. Cambridge, MA: Biogen, Inc;

Patient CH: Assessment/Plan �A: ◦ Since patient began Tecfidera® in 2013, no AE or change in disease state have been reported �P: ◦ Continue Tecfidera® 240 mg PO BID ◦ Continue regular monitoring with MS specialist ◦ Add additional symptomatic treatment if necessary

QUESTIONS?