Teacher Slide Lesson Plan 5 mins Elicit Recapping




- Slides: 18

Teacher Slide – Lesson Plan 5 mins – Elicit. Recapping particles diagrams to determine whether substances are elements or compound. 5 mins – Engage. Ask students why properties of elements vary so much given they are all made up of only one type of atom. Should lead to a discussion about them being made up of different atoms and then how atoms are different to each other to introduce the lesson. 10 mins – Explore. Students are to read through the particles in an atom sheet to establish the particles in an atom, what charge they have and where they are found. This should allow students to create a labelled diagram of the structure of an atom. Take feedback and clarify 10 mins – Explain. Inform students how to determine the number of each sub-atomic particles present in the structure of an atom. 5 mins – Evaluate. Check understanding so far by using mini whiteboards to attempt the ‘what am I? ’ task 15 mins –Elaborate. Students are to calculate the number of sub-atomic particles in an atom of an element for a range of different elements. Extension – Comparing the atomic structure of two different atoms (Fluorine and neon) 10 mins – Evaluate. Students are to peer assess each others work

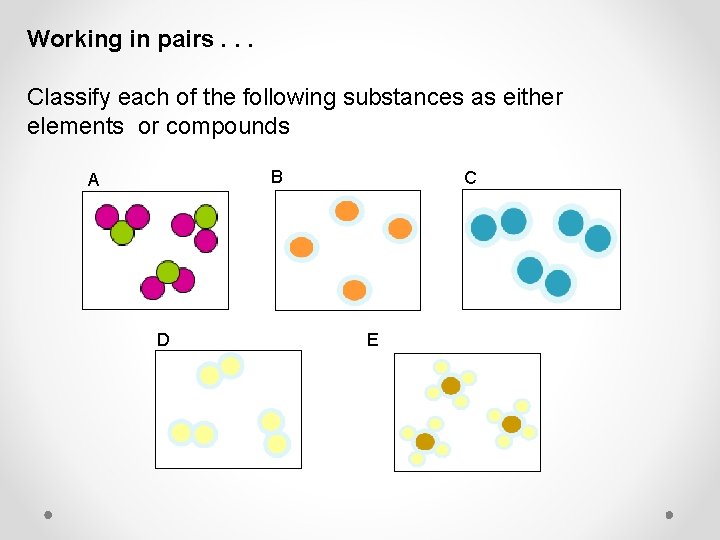
Working in pairs. . . Classify each of the following substances as either elements or compounds B A D C E

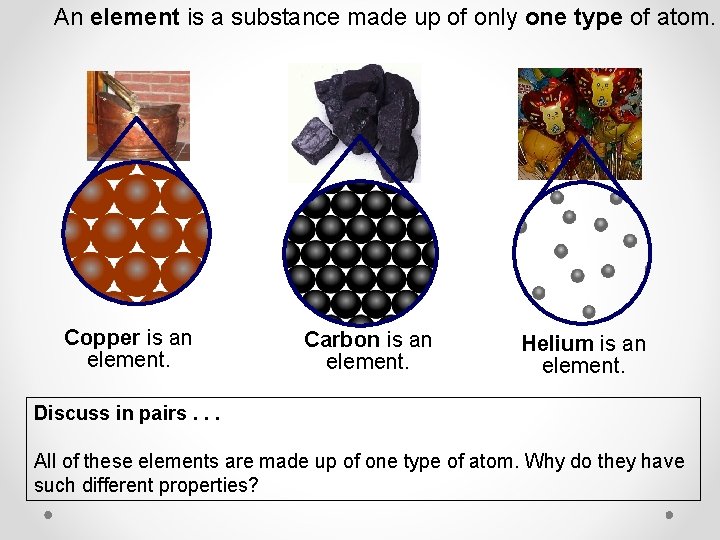
An element is a substance made up of only one type of atom. Copper is an element. Carbon is an element. Helium is an element. Discuss in pairs. . . All of these elements are made up of one type of atom. Why do they have such different properties?

12/01/2022 2. Structure of the Atom Learning Objective: To be able to determine the structure of the atom of an element ELEMENTS AND COMPOUNDS

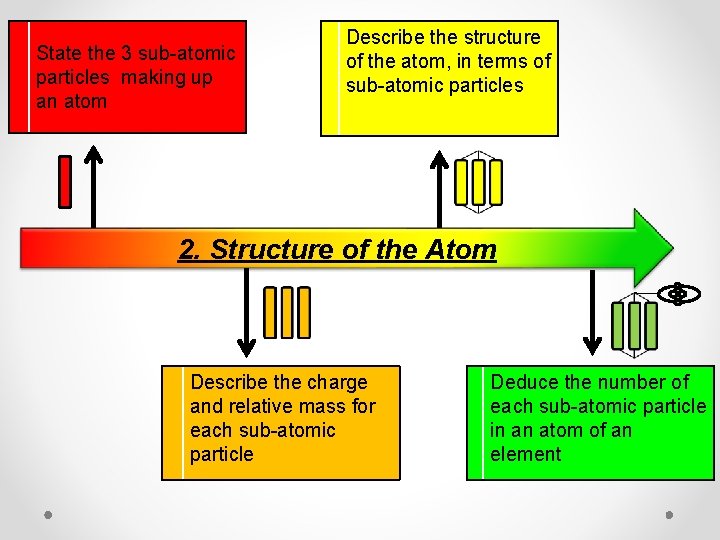
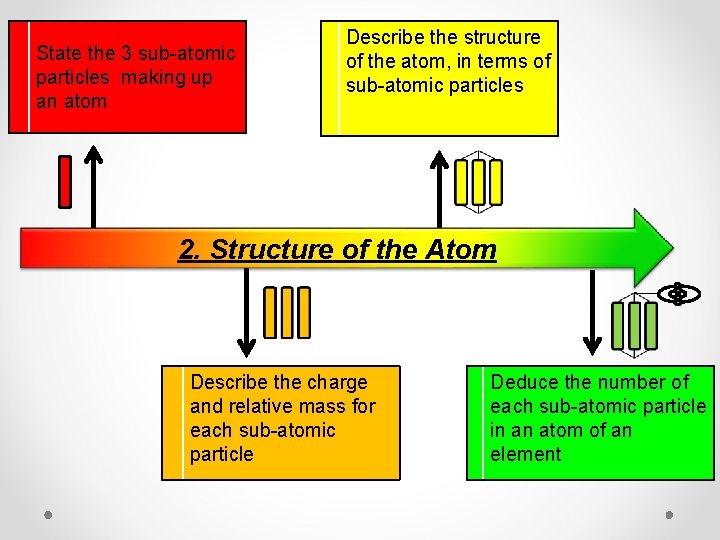
State the 3 sub-atomic particles making up an atom Describe the structure of the atom, in terms of sub-atomic particles 2. Structure of the Atom Describe the charge and relative mass for each sub-atomic particle Deduce the number of each sub-atomic particle in an atom of an element

The History of the Atom Dalton (1804) A simple sphere Developed ideas of Democritus about the atom by finding experimental evidence of their existence

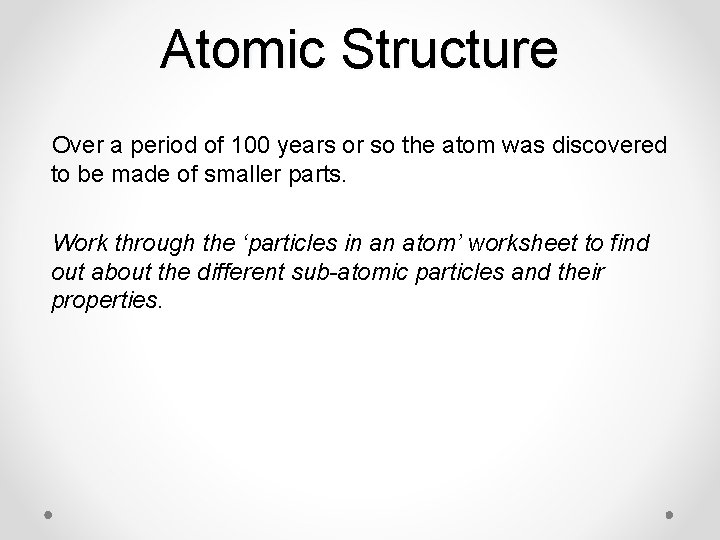
Atomic Structure Over a period of 100 years or so the atom was discovered to be made of smaller parts. Work through the ‘particles in an atom’ worksheet to find out about the different sub-atomic particles and their properties.

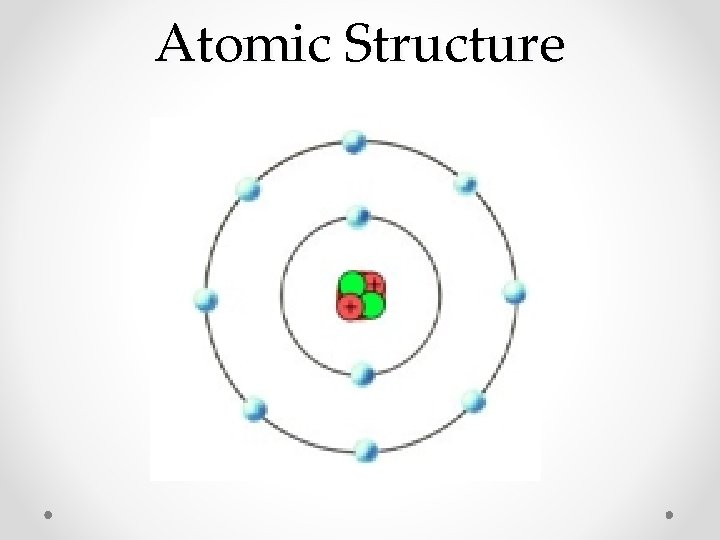
Atomic Structure

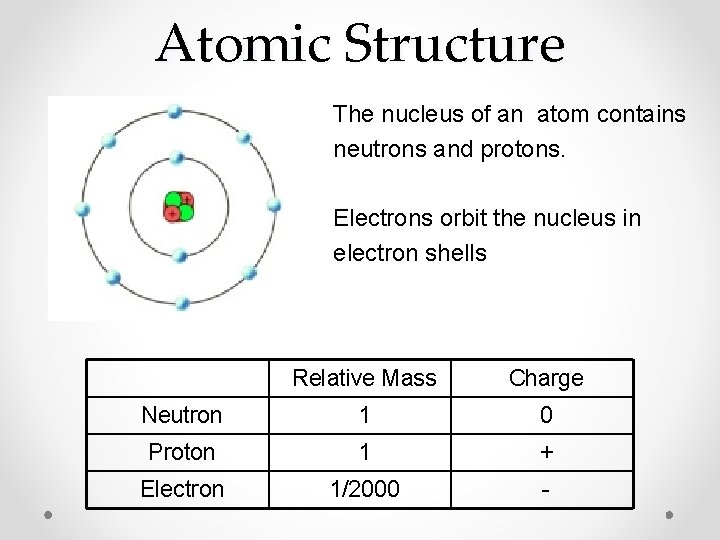
Atomic Structure The nucleus of an atom contains neutrons and protons. Electrons orbit the nucleus in electron shells Relative Mass Charge Neutron 1 0 Proton 1 + Electron 1/2000 -

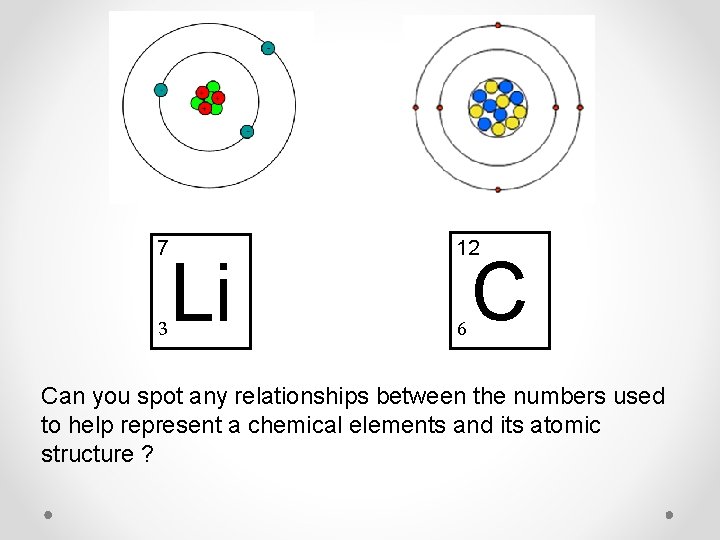
7 3 Li 12 6 C Can you spot any relationships between the numbers used to help represent a chemical elements and its atomic structure ?

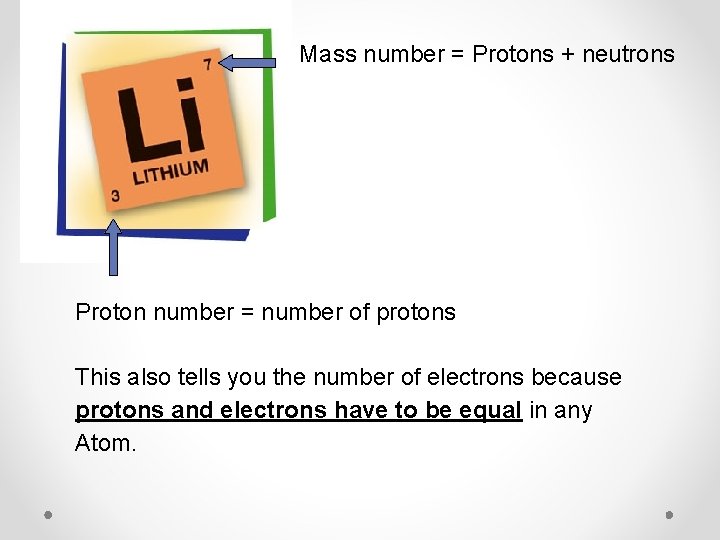
Mass number = Protons + neutrons Proton number = number of protons This also tells you the number of electrons because protons and electrons have to be equal in any Atom.

Periodic table - What am I? 1) I have four protons 2) I have a positive charge and spend my time in the nucleus 3) I have 7 electrons 4) I have 5 protons and 6 neutrons 5) I am not charged and spend my time around protons 6) I have 10 neutrons

Periodic table - What am I? 1) I have four protons - Beryllium 2) I have a positive charge and spend my time in the nucleus - Proton 3) I have 7 electrons - Nitrogen 4) I have 5 protons and 6 neutrons - Boron 5) I am not charged and spend my time around protons – Neutron 6) I have 10 neutrons – Fluorine or Neon

Element Helium Boron Magnesium Carbon Sulfur Fluorine Nitrogen Potassium Beryllium Hydrogen Protons Neutrons Electrons

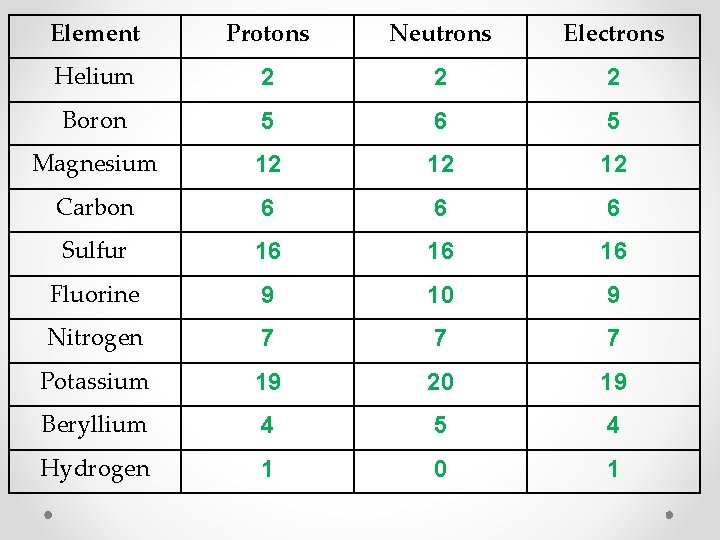
Element Protons Neutrons Electrons Helium 2 2 2 Boron 5 6 5 Magnesium 12 12 12 Carbon 6 6 6 Sulfur 16 16 16 Fluorine 9 10 9 Nitrogen 7 7 7 Potassium 19 20 19 Beryllium 4 5 4 Hydrogen 1 0 1

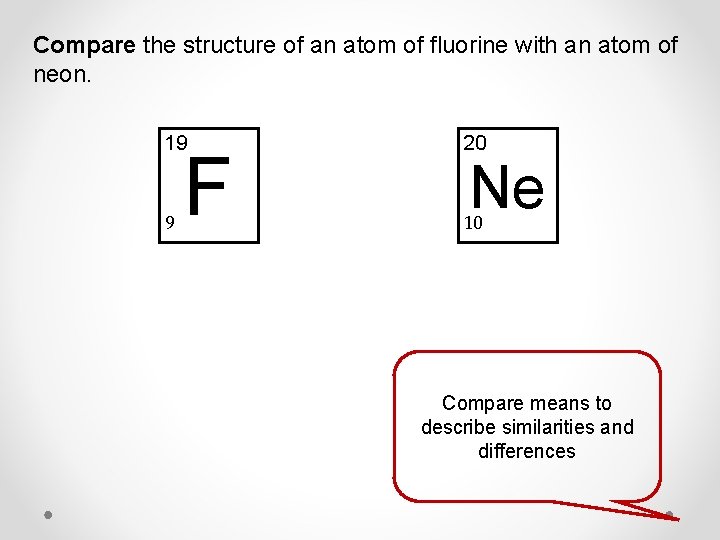
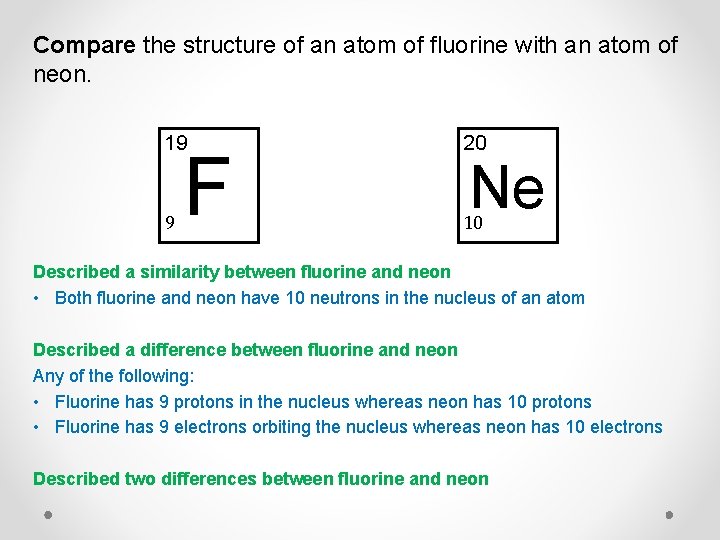
Compare the structure of an atom of fluorine with an atom of neon. 19 20 9 10 F Ne Compare means to describe similarities and differences

Compare the structure of an atom of fluorine with an atom of neon. 19 20 9 10 F Ne Described a similarity between fluorine and neon • Both fluorine and neon have 10 neutrons in the nucleus of an atom Described a difference between fluorine and neon Any of the following: • Fluorine has 9 protons in the nucleus whereas neon has 10 protons • Fluorine has 9 electrons orbiting the nucleus whereas neon has 10 electrons Described two differences between fluorine and neon

State the 3 sub-atomic particles making up an atom Describe the structure of the atom, in terms of sub-atomic particles 2. Structure of the Atom Describe the charge and relative mass for each sub-atomic particle Deduce the number of each sub-atomic particle in an atom of an element