Targeting FGFR Signaling in Cancer Bladder and Beyond

Targeting FGFR Signaling in Cancer: Bladder and Beyond Ignacio Durán, MD, Ph. D Associate Professor GU Oncology Program Leader Department of Medical Oncology Hospital Universitario Marques de Valdecilla-Idival Santander, Spain Supported by an educational grant from Janssen Pharmaceutica NV.
About These Slides § Please feel free to use, update, and share some or all of these slides in your noncommercial presentations to colleagues or patients § When using our slides, please retain the source attribution: Slide credit: clinicaloptions. com § These slides may not be published, posted online, or used in commercial presentations without permission. Please contact permissions@clinicaloptions. com for details

Faculty Disclosures Ignacio Durán, MD, Ph. D, has disclosed that he has received consulting fees from Astellas, Bristol-Myers Squibb, Ipsen, Jansen, Merck, MSD, Pharmacyclics, Roche/Genentech, and Seattle Genetics; fees for non. CME/CE services from Bayer, Bristol-Myers Squibb, EUSA Pharma, Ipsen, Jansen, Roche/Genentech; and other financial support from Astra. Zeneca.
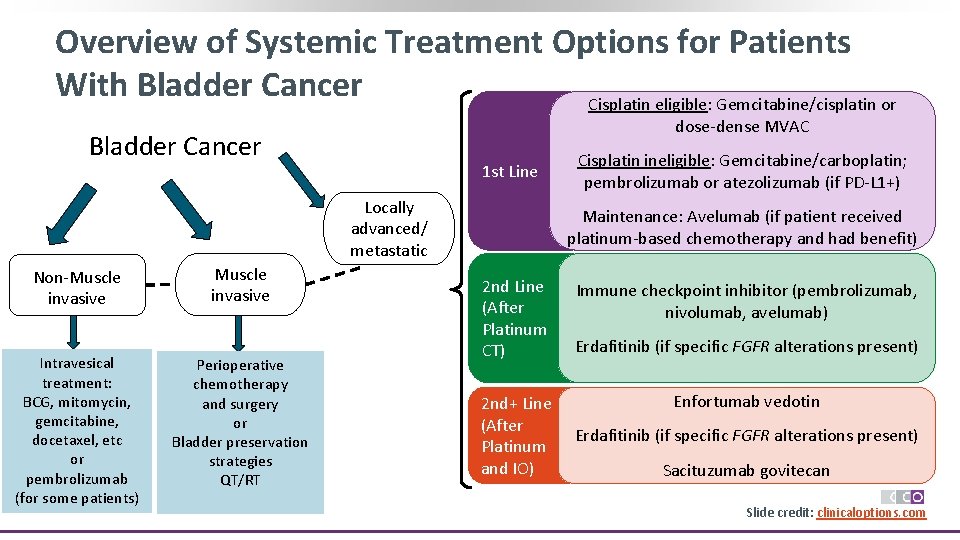
Overview of Systemic Treatment Options for Patients With Bladder Cancer Cisplatin eligible: Gemcitabine/cisplatin or dose-dense MVAC Bladder Cancer 1 st Line Locally advanced/ metastatic Non-Muscle invasive Intravesical treatment: BCG, mitomycin, gemcitabine, docetaxel, etc or pembrolizumab (for some patients) Muscle invasive Perioperative chemotherapy and surgery or Bladder preservation strategies QT/RT Cisplatin ineligible: Gemcitabine/carboplatin; pembrolizumab or atezolizumab (if PD-L 1+) Maintenance: Avelumab (if patient received platinum-based chemotherapy and had benefit) 2 nd Line (After Platinum CT) Immune checkpoint inhibitor (pembrolizumab, nivolumab, avelumab) Erdafitinib (if specific FGFR alterations present) Enfortumab vedotin 2 nd+ Line (After Erdafitinib (if specific FGFR alterations present) Platinum CT and IO) Sacituzumab govitecan Slide credit: clinicaloptions. com
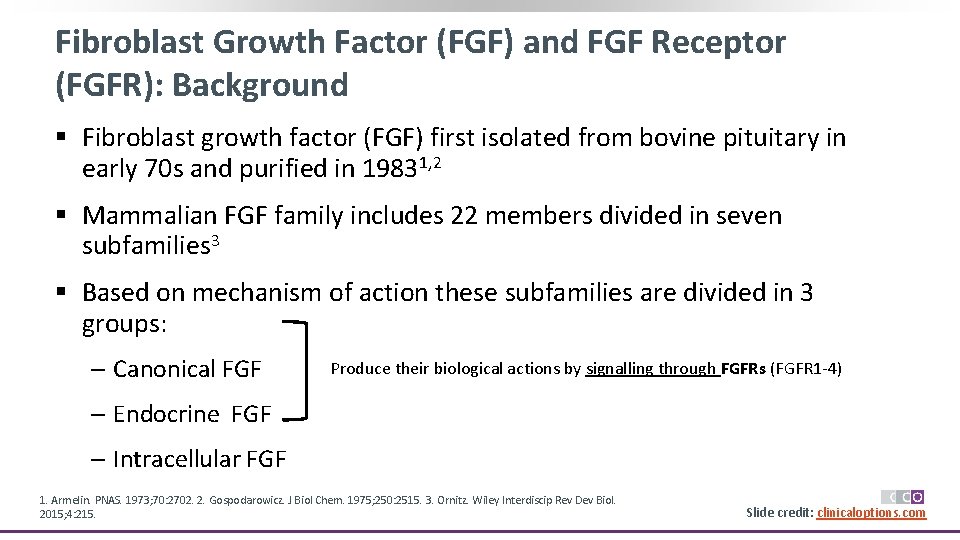
Fibroblast Growth Factor (FGF) and FGF Receptor (FGFR): Background § Fibroblast growth factor (FGF) first isolated from bovine pituitary in early 70 s and purified in 19831, 2 § Mammalian FGF family includes 22 members divided in seven subfamilies 3 § Based on mechanism of action these subfamilies are divided in 3 groups: ‒ Canonical FGF Produce their biological actions by signalling through FGFRs (FGFR 1 -4) ‒ Endocrine FGF ‒ Intracellular FGF 1. Armelin. PNAS. 1973; 70: 2702. 2. Gospodarowicz. J Biol Chem. 1975; 250: 2515. 3. Ornitz. Wiley Interdiscip Rev Dev Biol. 2015; 4: 215. Slide credit: clinicaloptions. com

FGFR: Fibroblast Growth Factor Receptor § FGFR: membrane-based tyrosine kinase receptor involved in cell proliferation, differentiation, and steroid biosynthesis 1 § FGFR drives malignancy by enhanced kinase domain activation, ligandindependent receptor dimerization, or altered FGF ligand affinity, gene amplifications, or gene fusions § FGFR mutations (R 248 C, S 249 C, G 370 C, Y 373 C), overexpression implicated in bladder cancer 2 § FGFR inhibitors and anti-FGFR ADCs are in ongoing and upcoming trials in advanced UC 1. Wu. Nat Rev Cancer. 2005; 5: 713; 2. Turo. J Urol. 2015; 193: 325. Slide credit: clinicaloptions. com

FGF–FGFR Interaction and Signaling Pathways FGF-FGFR Signaling Pathway 6 § FGFR TK domains have ATP-binding area and phosphorylating tyrosine residues 1 § Heparin and heparan sulphate proteoglycans (HSPG) are essential cofactors for binding canonical FGFs 2 § Binding of FGFs leads to dimerization of FGFRs and activation of 4 major intracellular signaling pathways: STATs, Ras-Raf-MAPK, PI 3 K-AKT and PLCγ 3 § FGF-FGFR signaling participates in key cell behaviors (eg, proliferation, differentiation, survival, migration, and angiogenesis)3, 4 § Endocrine FGFs can regulate bile acid metabolism, lipid metabolism, and phosphate and vitamin D levels in serum 3, 5, 6 1. Farrell. Biochem Soc Trans. 2018; 46: 1753. 2. Huhtala. Structure. 1999; 7: 699. 3. Ornitz. Wiley Interdiscip Rev Dev Biol. 2015; 4: 215. 4. Turner. Nature Reviews Cancer. 2010; 116. 5. Itoh. Front Endocrinol (Lausanne). 2015; 6: 154. 6. Liu. Cell Prolif. 2021; 54: e 13009. Figure 1 of given citation is used in its original form under the terms and conditions of the Creative Commons Attribution 4. 0 International license (CC BY 4. 0: https: //creativecommons. org/licenses/by/4. 0/). Slide credit: clinicaloptions. com
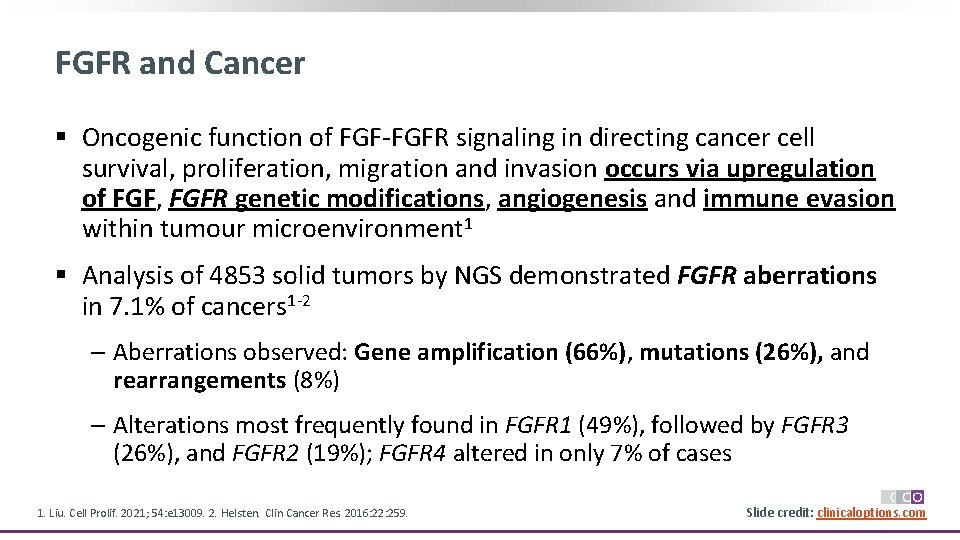
FGFR and Cancer § Oncogenic function of FGF-FGFR signaling in directing cancer cell survival, proliferation, migration and invasion occurs via upregulation of FGF, FGFR genetic modifications, angiogenesis and immune evasion within tumour microenvironment 1 § Analysis of 4853 solid tumors by NGS demonstrated FGFR aberrations in 7. 1% of cancers 1 -2 ‒ Aberrations observed: Gene amplification (66%), mutations (26%), and rearrangements (8%) ‒ Alterations most frequently found in FGFR 1 (49%), followed by FGFR 3 (26%), and FGFR 2 (19%); FGFR 4 altered in only 7% of cases 1. Liu. Cell Prolif. 2021; 54: e 13009. 2. Helsten. Clin Cancer Res. 2016: 22: 259. Slide credit: clinicaloptions. com
Ligand-Independent Mechanisms of FGFR Activation Figure not available Babina. Nat Rev Cancer. 2017; 17: 318. § FGFR gene amplification commonly leads to protein overexpression, leading to increased receptor accumulation and activation of the downstream signaling pathways § Activating mutations result in increased dimerization of receptors in absence of ligand or constitutive kinase domain activation § Chromosomal translocations result in fusion with genes encoding other proteins that increase dimerization of receptors or lead to receptor hyperactivation in a ligandindependent manner § FGFs can be produced by the tumor cells or by cells in the stromal compartment or autocrine activation through splicing mechanism § FGFs may also contribute to angiogenesis or epithelial– mesenchymal transition § Deregulation of other partners such as FRS 2 or PLCγ might lead to hyperactivation of FGFR pathway Slide credit: clinicaloptions. com

FGFR Somatic Mutation Frequencies and Locations § Mutations in FGFR 1 and FGFR 4 NOT frequently reported Figure not available Babina. Nat Rev Cancer. 2017; 17: 318. § Mutations in FGFR 2 and FGFR 3 common and occur predominantly in ligandbinding and TM domains, with fewer mutations reported in kinase domains Slide credit: clinicaloptions. com

FGFR Gene Fusions FGFR 3 -TACC 3 Gene Fusion 2 Chromosome 4 p 16. 3 § Various FGFR gene fusions can lead to inconsistent expression of fusion proteins containing a transcription factor and TKs; can cause ligand-independent receptor dimerization and oncogenic effects 1 § FGFR 3 -TACC 3: an oncogene present in urothelial carcinoma and other tumors TACC 3 FGFR 3 Duplication and Insertion ‒ fused protein phosphorylates phosphopeptide PIN 4 and promotes tumor growth and triggers MAPK-ERK and JAK-STAT signaling pathways FGFR 3 TACC 3 1. Liu. Cell Prolif. 2021; 54: e 13009. 2. Costa. Oncotarget. 2016; 7: 55924. Figure 2 of given citation is used in its original form under the terms and conditions of the Creative Commons Attribution 4. 0 International license (CC BY 4. 0: https: //creativecommons. org/licenses/by/4. 0/). Slide credit: clinicaloptions. com
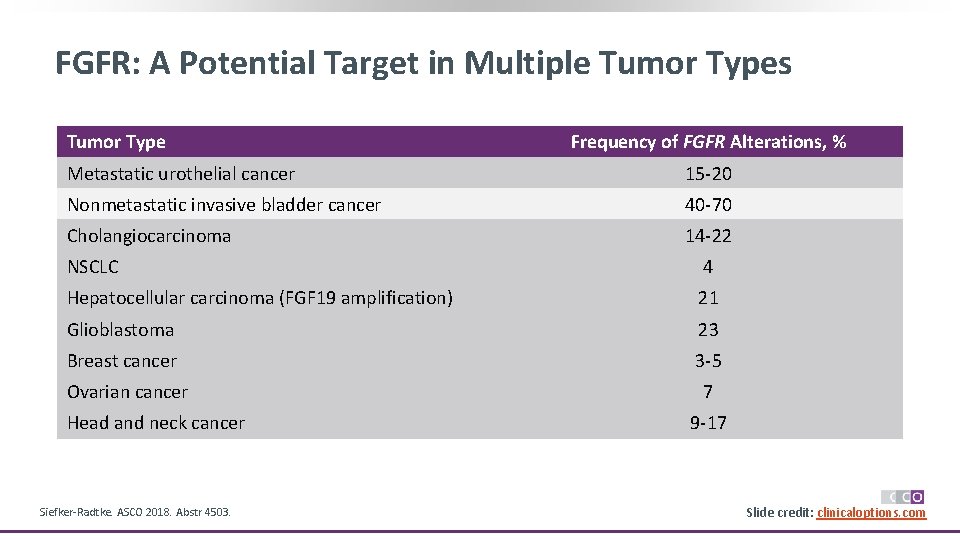
FGFR: A Potential Target in Multiple Tumor Types Tumor Type Frequency of FGFR Alterations, % Metastatic urothelial cancer 15 -20 Nonmetastatic invasive bladder cancer 40 -70 Cholangiocarcinoma 14 -22 NSCLC 4 Hepatocellular carcinoma (FGF 19 amplification) 21 Glioblastoma 23 Breast cancer 3 -5 Ovarian cancer 7 Head and neck cancer Siefker-Radtke. ASCO 2018. Abstr 4503. 9 -17 Slide credit: clinicaloptions. com
Targeting FGF-FGFR For Cancer Treatment § Role of FGF-FGFR signalling in tumorigenesis lead to development of several drugs targeting this signalling pathway § Drugs classified into different categories according to their action mechanism : ‒ SMALL-MOLECULE FGFR TKIs: Divided into selective and non-selective according to whether IC 50 of inhibitory activity to other kinases is <10 n. M ‒ ANTI-FGFR ANTIBODIES: Targeting different FGFRs ‒ FGF LIGAND TRAPS: Decoys ‒ ANTIBODY-DRUG CONJUGATES: Antibody with a payload Babina. Nat Rev Cancer. 2017; 17: 318. Slide credit: clinicaloptions. com
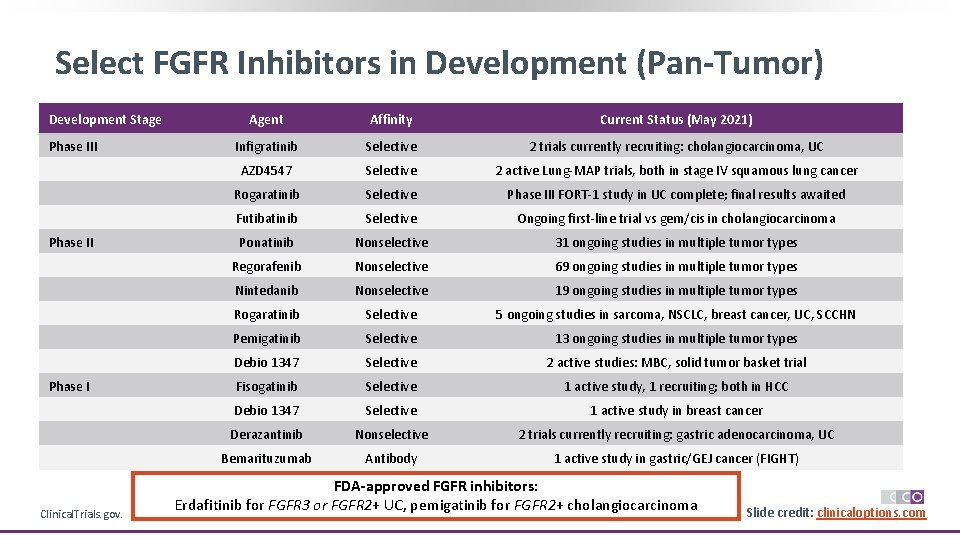
Select FGFR Inhibitors in Development (Pan-Tumor) Development Stage Phase III Phase I Clinical. Trials. gov. Agent Affinity Current Status (May 2021) Infigratinib Selective 2 trials currently recruiting: cholangiocarcinoma, UC AZD 4547 Selective 2 active Lung-MAP trials, both in stage IV squamous lung cancer Rogaratinib Selective Phase III FORT-1 study in UC complete; final results awaited Futibatinib Selective Ongoing first-line trial vs gem/cis in cholangiocarcinoma Ponatinib Nonselective 31 ongoing studies in multiple tumor types Regorafenib Nonselective 69 ongoing studies in multiple tumor types Nintedanib Nonselective 19 ongoing studies in multiple tumor types Rogaratinib Selective 5 ongoing studies in sarcoma, NSCLC, breast cancer, UC, SCCHN Pemigatinib Selective 13 ongoing studies in multiple tumor types Debio 1347 Selective 2 active studies: MBC, solid tumor basket trial Fisogatinib Selective 1 active study, 1 recruiting; both in HCC Debio 1347 Selective 1 active study in breast cancer Derazantinib Nonselective 2 trials currently recruiting: gastric adenocarcinoma, UC Bemarituzumab Antibody 1 active study in gastric/GEJ cancer (FIGHT) FDA-approved FGFR inhibitors: Erdafitinib for FGFR 3 or FGFR 2+ UC, pemigatinib for FGFR 2+ cholangiocarcinoma Slide credit: clinicaloptions. com
Monoclonal Antibodies Targeting FGFR § Function via several mechanisms: Disruption of ligand binding and/or receptor dimerization or conjugation of the antibody of interest to a cytotoxic agent (ADCs) § VOFATAMAB (B-701)1, 2 ‒ Fully human m. Ab targeting FGFR 3 that blocks activation of wildtype and mutant receptors ‒ FIERCE-21 phase Ib/II study: Well-tolerated in combination with docetaxel in patients with m. UC with most TEAEs being grade 2 or less; FIERCE-22 phase Ib/II study: Combined vofatamab plus pembrolizumab in patients with m. UC showed benefit—even in FGFR 3 WT patients § BEMARITUZUMAB (FPA 144)3, 4 ‒ First-in-class humanized Ig. G 1 m. Ab specific to splice-variant FGFR 2 b; inhibits binding of FGF 7, FGF 10, and FGF 22 but not FGF 23 which is required for phosphate and vitamin D metabolism; enhances ADCC § MGFR 1877 S 5 ‒ Binds to FGFR 3 to competitively inhibit native ligand binding and prevent receptor dimerization in cells with wild-type or mutant FGFR 3 1. Necchi. ASCO 2019. Abstr 409. 2. Siefker-Radtke. ASCO 2019. Abstr 4511. 3. Catenacci. JCO. 2020 Jul 20; 38(21): 2418. 4. Catenacci. Future Oncol. 2019; 15: 2073. 5. Trudel. ASH 2012. Abstr 4029. Slide credit: clinicaloptions. com
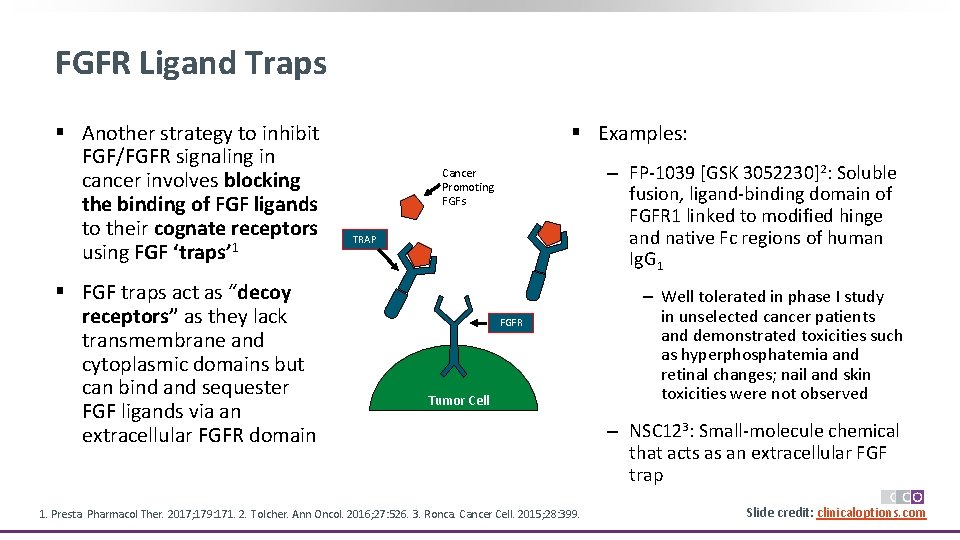
FGFR Ligand Traps § Another strategy to inhibit FGF/FGFR signaling in cancer involves blocking the binding of FGF ligands to their cognate receptors using FGF ‘traps’ 1 § FGF traps act as “decoy receptors” as they lack transmembrane and cytoplasmic domains but can bind and sequester FGF ligands via an extracellular FGFR domain § Examples: ‒ FP-1039 [GSK 3052230]2: Soluble fusion, ligand-binding domain of FGFR 1 linked to modified hinge and native Fc regions of human Ig. G 1 Cancer Promoting FGFs TRAP FGFR Tumor Cell 1. Presta. Pharmacol Ther. 2017; 179: 171. 2. Tolcher. Ann Oncol. 2016; 27: 526. 3. Ronca. Cancer Cell. 2015; 28: 399. ‒ Well tolerated in phase I study in unselected cancer patients and demonstrated toxicities such as hyperphosphatemia and retinal changes; nail and skin toxicities were not observed ‒ NSC 123: Small-molecule chemical that acts as an extracellular FGF trap Slide credit: clinicaloptions. com
Antibody-Drug Conjugates (ADC) Targeting FGFR § BAY 1187982 (aprutumab ixadotin)1 ‒ Uses derivative of highly potent microtubule-disrupting agent auristatin and is selective for the FGFR 2 -IIIb and FGFR 2 -IIIc isoforms ‒ Interesting pre-clinical data but failed in clinical development § LY 30762262 ‒ FGFR 3 - specific ADC conjugated to cytotoxic agent DM 4 (ravtansine), a maytansine derivative ‒ Interesting activity in preclinical FGFR 3–TACC 3 fusion and G 370 C, S 249 C or R 248 C FGFR 3 mutant models; results pending from completed phase I trial targeting FGFR 1. Kim. Target Oncol. 2019; 14: 591. 2. Surguladze. ACCR 2019. Abstr 4835. Slide credit: clinicaloptions. com
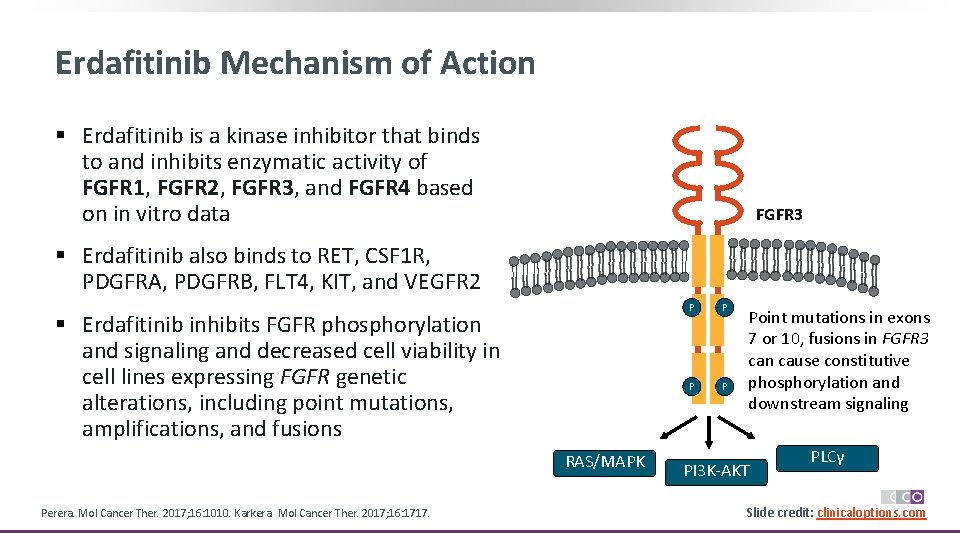
Erdafitinib Mechanism of Action § Erdafitinib is a kinase inhibitor that binds to and inhibits enzymatic activity of FGFR 1, FGFR 2, FGFR 3, and FGFR 4 based on in vitro data FGFR 3 § Erdafitinib also binds to RET, CSF 1 R, PDGFRA, PDGFRB, FLT 4, KIT, and VEGFR 2 § Erdafitinib inhibits FGFR phosphorylation and signaling and decreased cell viability in cell lines expressing FGFR genetic alterations, including point mutations, amplifications, and fusions RAS/MAPK Perera. Mol Cancer Ther. 2017; 16: 1010. Karkera. Mol Cancer Ther. 2017; 16: 1717. P P Point mutations in exons 7 or 10, fusions in FGFR 3 can cause constitutive phosphorylation and downstream signaling PI 3 K-AKT PLCγ Slide credit: clinicaloptions. com
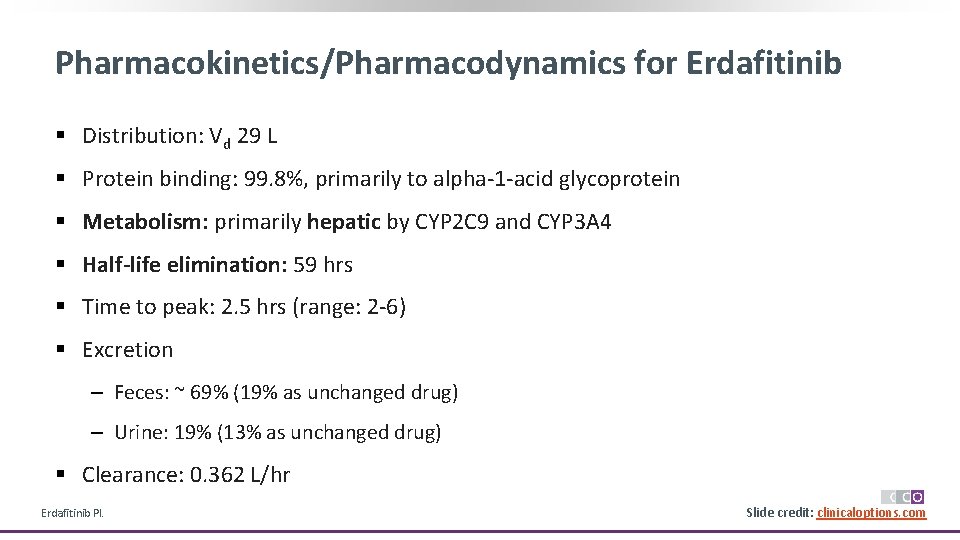
Pharmacokinetics/Pharmacodynamics for Erdafitinib § Distribution: Vd 29 L § Protein binding: 99. 8%, primarily to alpha-1 -acid glycoprotein § Metabolism: primarily hepatic by CYP 2 C 9 and CYP 3 A 4 § Half-life elimination: 59 hrs § Time to peak: 2. 5 hrs (range: 2 -6) § Excretion ‒ Feces: ~ 69% (19% as unchanged drug) ‒ Urine: 19% (13% as unchanged drug) § Clearance: 0. 362 L/hr Erdafitinib PI. Slide credit: clinicaloptions. com
Erdafitinib in FGFR-Altered Urothelial Carcinoma (BLC 2001): Background § In patients with advanced UC, second-line single-agent CT with vinflunine or taxanes historically associated with ORR of ~ 10% and median OS of 7 -9 mos[1, 2] § More therapy options needed in this setting because while outcomes have improved with PD-1/PDL 1 checkpoint inhibitors (ORR ~ 15 -20%; m. OS ~ 10 mos), many patients do not derive benefit [3, 4] ‒ Response to immunotherapy varies by TCGA subtype[5] ‒ Luminal I subtype of UC can be immunologically “cold” and less responsive to checkpoint inhibitors [6] § FGFR altered in 15 -20% of advanced UC cases (mutated FGFR 3 in 54% of upper tract UC)[7, 8] § Erdafitinib (JNJ-42756493): oral pan-FGFR inhibitor with IC 50 in low nanomolar range for FGFR 1 -4[9] ‒ Durable inhibitory activity may be related to sustained intracellular release after taken up by lysosomes ‒ Associated with antitumor activity in FGFR-altered advanced UC and other tumor types[10, 11] 1. Mc. Caffrey. JCO. 1997; 15: 1853. 2. Bellmunt. JCO. 2009; 27: 4454. 3. Powles. Lancet. 2018; 391: 748. 4. Bellmunt. NEJM. 2017; 376: 1015. 5. Sharma. Lancet Oncol. 2017; 18: 312. 6. Siefker-Radtke. J Urol. 2018; 199: 1129. 7. Rodriguez-Vida. J Hematol Oncol. 2015; 8: 119. 8. Li. Curr Urol Rep. 2016; 17: 12. 9. Perera. Mol Cancer Ther. 2017; 16: 1010. Tabernero. JCO. 2015; 33: 3401. 11. Soria. ESMO 2016. Abstr 781 PD. Slide credit: clinicaloptions. com
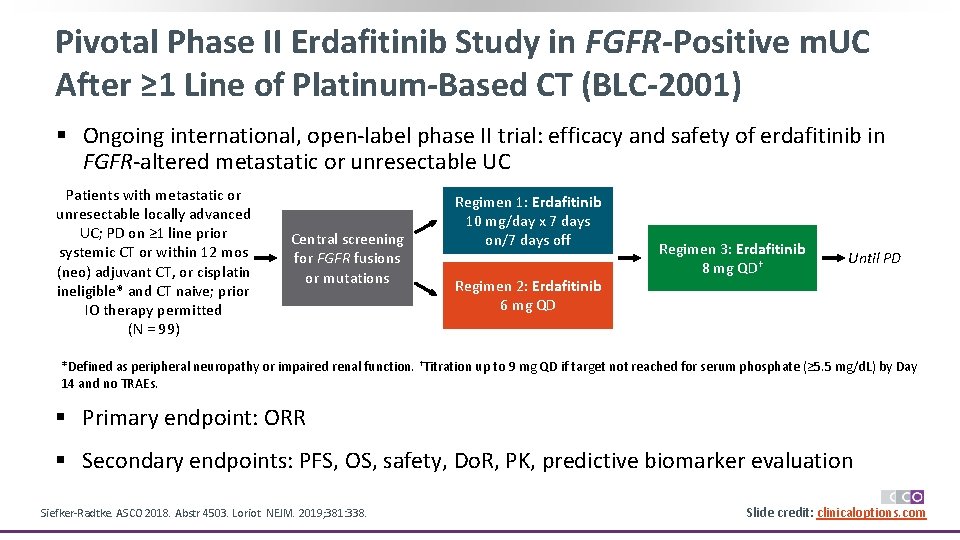
Pivotal Phase II Erdafitinib Study in FGFR-Positive m. UC After ≥ 1 Line of Platinum-Based CT (BLC-2001) § Ongoing international, open-label phase II trial: efficacy and safety of erdafitinib in FGFR-altered metastatic or unresectable UC Patients with metastatic or unresectable locally advanced UC; PD on ≥ 1 line prior systemic CT or within 12 mos (neo) adjuvant CT, or cisplatin ineligible* and CT naive; prior IO therapy permitted (N = 99) Central screening for FGFR fusions or mutations Regimen 1: Erdafitinib 10 mg/day x 7 days on/7 days off Regimen 2: Erdafitinib 6 mg QD Regimen 3: Erdafitinib 8 mg QD† Until PD *Defined as peripheral neuropathy or impaired renal function. †Titration up to 9 mg QD if target not reached for serum phosphate (≥ 5. 5 mg/d. L) by Day 14 and no TRAEs. § Primary endpoint: ORR § Secondary endpoints: PFS, OS, safety, Do. R, PK, predictive biomarker evaluation Siefker-Radtke. ASCO 2018. Abstr 4503. Loriot. NEJM. 2019; 381: 338. Slide credit: clinicaloptions. com
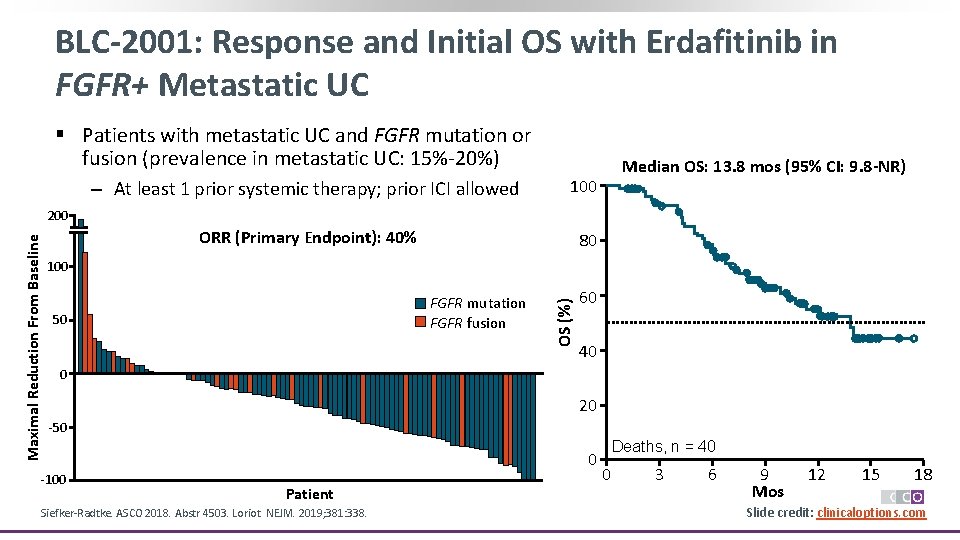
BLC-2001: Response and Initial OS with Erdafitinib in FGFR+ Metastatic UC § Patients with metastatic UC and FGFR mutation or fusion (prevalence in metastatic UC: 15%-20%) ‒ At least 1 prior systemic therapy; prior ICI allowed 100 ORR (Primary Endpoint): 40% 80 Median OS: 13. 8 mos (95% CI: 9. 8 -NR) 100 FGFR mutation FGFR fusion 50 OS (%) Maximal Reduction From Baseline 200 60 40 0 20 -50 -100 0 Patient Siefker-Radtke. ASCO 2018. Abstr 4503. Loriot. NEJM. 2019; 381: 338. Deaths, n = 40 0 3 6 9 Mos 12 15 18 Slide credit: clinicaloptions. com
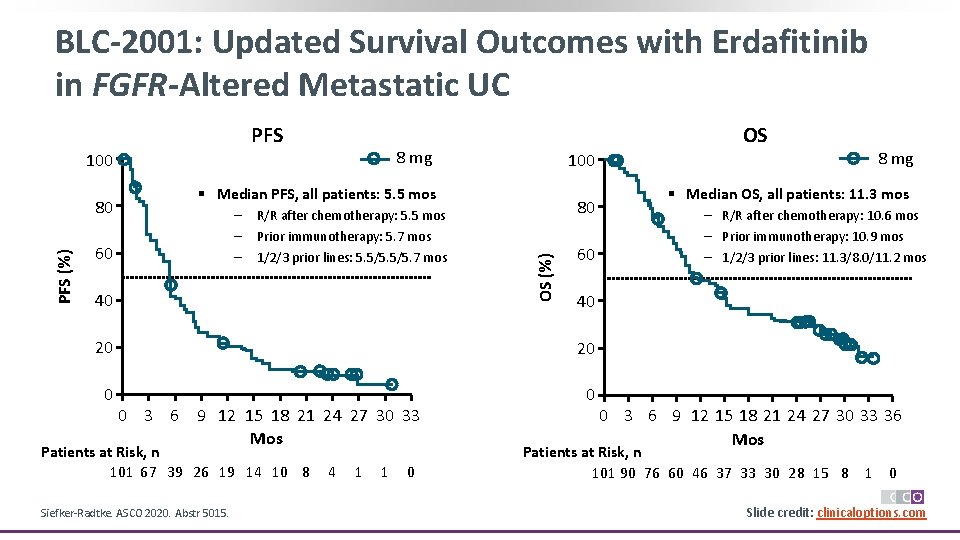
BLC-2001: Updated Survival Outcomes with Erdafitinib in FGFR-Altered Metastatic UC PFS 8 mg 100 § Median PFS, all patients: 5. 5 mos – – – 60 R/R after chemotherapy: 5. 5 mos Prior immunotherapy: 5. 7 mos 1/2/3 prior lines: 5. 5/5. 7 mos 40 80 OS (%) PFS (%) 80 OS 60 20 0 0 Patients at Risk, n 101 67 39 26 19 14 10 8 Siefker-Radtke. ASCO 2020. Abstr 5015. 4 1 1 0 § Median OS, all patients: 11. 3 mos – R/R after chemotherapy: 10. 6 mos – Prior immunotherapy: 10. 9 mos – 1/2/3 prior lines: 11. 3/8. 0/11. 2 mos 40 20 0 3 6 9 12 15 18 21 24 27 30 33 Mos 8 mg 0 3 6 9 12 15 18 21 24 27 30 33 36 Mos Patients at Risk, n 101 90 76 60 46 37 33 30 28 15 8 1 0 Slide credit: clinicaloptions. com
Erdafitinib Approval § In April 2019, FDA granted accelerated approval to erdafitinib for locally advanced/metastatic bladder cancer with susceptible FGFR 2 or FGFR 3 gene alterations, after platinum chemotherapy (including within 12 mos of neoadjuvant or adjuvant) § Erdafitinib is an oral potent pan-FGFR (1 -4) inhibitor § Single-digit nanomolar range molecule (ie, high binding affinity) ‒ Erdafitinib is taken up by lysosomes, resulting in intracellular release, which may contribute to its long-lasting activity § Approval is based on FGFR genetic alterations (including certain FGFR 3 gene mutations or FGFR 2 or FGFR 3 gene fusions) in tumor specimens detected by an FDA-approved companion diagnostic therascreen FGFR RGQ RT-PCR Kit developed Slide credit: clinicaloptions. com
Considerations for Use of Erdafitinib in Clinical Practice § Does patient have an FGFR 2/3 alteration? ‒ Necessary for use of erdafitinib § What have prior therapies been, and what was response? ‒ Enfortumab vedotin approved for use after platinum and IO § Any residual toxicities of prior therapies? ‒ Eg, neuropathy § Patient comorbidities? ‒ Keeping in mind, median age at diagnosis = 73 yrs, most 60 -85 yrs ‒ Baseline assessment of functional status ‒ Baseline comorbid diseases (eg, diabetes, ocular issues, dermatologic issues) Slide credit: clinicaloptions. com
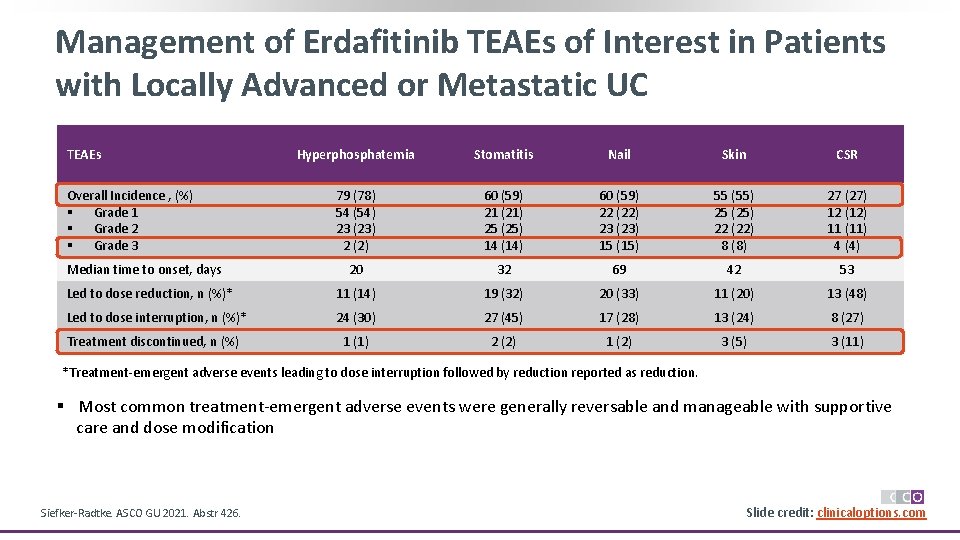
Management of Erdafitinib TEAEs of Interest in Patients with Locally Advanced or Metastatic UC TEAEs Hyperphosphatemia Stomatitis Nail Skin CSR 79 (78) 54 (54) 23 (23) 2 (2) 60 (59) 21 (21) 25 (25) 14 (14) 60 (59) 22 (22) 23 (23) 15 (15) 55 (55) 25 (25) 22 (22) 8 (8) 27 (27) 12 (12) 11 (11) 4 (4) 20 32 69 42 53 Led to dose reduction, n (%)* 11 (14) 19 (32) 20 (33) 11 (20) 13 (48) Led to dose interruption, n (%)* 24 (30) 27 (45) 17 (28) 13 (24) 8 (27) Treatment discontinued, n (%) 1 (1) 2 (2) 1 (2) 3 (5) 3 (11) Overall Incidence , (%) § Grade 1 § Grade 2 § Grade 3 Median time to onset, days *Treatment-emergent adverse events leading to dose interruption followed by reduction reported as reduction. § Most common treatment-emergent adverse events were generally reversable and manageable with supportive care and dose modification Siefker-Radtke. ASCO GU 2021. Abstr 426. Slide credit: clinicaloptions. com
Hyperphosphatemia and Erdafitinib Therapy § FGFR inhibition counteracts renal FGF-23/Klotho signaling, resulting in CYP 27 B 1 and CYP 24 A 1 deregulation and hypervitaminosis D and hyperphosphatemia induction § Mean phosphate levels peaked across doses and schedules between Day 7/8 and Day 35/36 § Median time to hyperphosphatemia onset (any grade): 20 days (range: 8 -116 days); 32% of patients received phosphate binders during erdafitinib treatment § Avoid concomitant use with agents that may increase serum phosphate levels (eg, potassium phosphate or vitamin D supplements, antacids, phosphate-containing enemas or laxatives, and other medications with phosphate excipients) before the initial (Days 1421) dose increase period (based on serum phosphate levels) § Hyperphosphatemia may require treatment interruption, dosage adjustment, and/or the use of phosphate binders Slide credit: clinicaloptions. com
Ocular Toxicity and Erdafitinib Therapy § Central serous retinopathy/retinal pigment epithelial detachment reported in ~ 25% of patients; may result in visual field defect, including central field of vision ‒ Median time to first onset of CSR/RPED: 50 days ‒ Resolution occurred in 13% of patients; persistent CSR/RPED was reported in another 13% of patients ‒ Temporarily interrupt erdafitinib if CSR/RPED develops; permanently discontinue if toxicity does not resolve within 4 wks or for grade 4 toxicity § Dry eye symptoms also occurred in 28% of patients, including grade 3 events; administer dry eye prophylaxis (with ocular demulcents) as needed § Ocular toxicity may require therapy interruption, dosage adjustment, and/or therapy discontinuation Slide credit: clinicaloptions. com
Managing Patients on Erdafitinib Clinical Pearls § Establish means of communication to respond to lab changes and AEs dose interruption and/or reduction as indicated § Stomatitis/dry mouth: S+S rinses, saliva stimulant, “magic” mouthwash, consider dexamethasone mouthwash § Nail and skin changes: emollients, podiatry care, prevent infection § Diarrhea: antidiarrheal agents § Dry eyes: “artificial tears, ” lubricants—referral to ophthalmologist § CSR: as per ophthalmologist with dose interruption/dose reduction Erdafitinib PI. Slide credit: clinicaloptions. com

Erdafitinib Dose and Administration Starting dose of 8 mg orally daily; up titrate to 9 mg daily if criteria met; if serum phosphate level is <5. 5 mg/d. L and there are no ocular disorders or grade ≥ 2 adverse events after 14 -21 days of treatment initiation § Dosage forms: 3 -mg, 4 -mg, and 5 -mg tablets § Administration: swallow tablets whole, with or without food ‒ If vomiting occurs any time after taking the drug, the next dose should be taken the next day ‒ If a dose is missed, it can be taken as soon as possible on the same day; resume the regular daily dose schedule the next day; extra tablets should not be taken to make up for the missed dose § Dietary considerations: restrict phosphate intake to 600 -800 mg daily Erdafitinib PI. Slide credit: clinicaloptions. com
Patient Monitoring While Receiving Erdafitinib § Monitor serum phosphate level at baseline, at 14 -21 days after therapy initiation, and then monthly or as clinically necessary § Ophthalmologic exams at baseline, monthly for the first 4 mos of erdafitinib therapy, then every 3 mos thereafter and as clinically necessary ‒ Exam should include visual acuity assessment, slit lamp examination, fundoscopy, and optical coherence tomography § Evaluate pregnancy status prior to use in females of reproductive potential because of embryo-fetal toxicity warning. ‒ Advise female patients of reproductive potential and male patients with female partners of reproductive potential to use effective contraception during treatment and for one month after the last dose ‒ Because of the potential for serious adverse events from erdafitinib in a breastfed child, advise lactating women not to breastfeed during treatment and for 1 mo following the last dose § Monitor adherence Erdafitinib PI. Slide credit: clinicaloptions. com
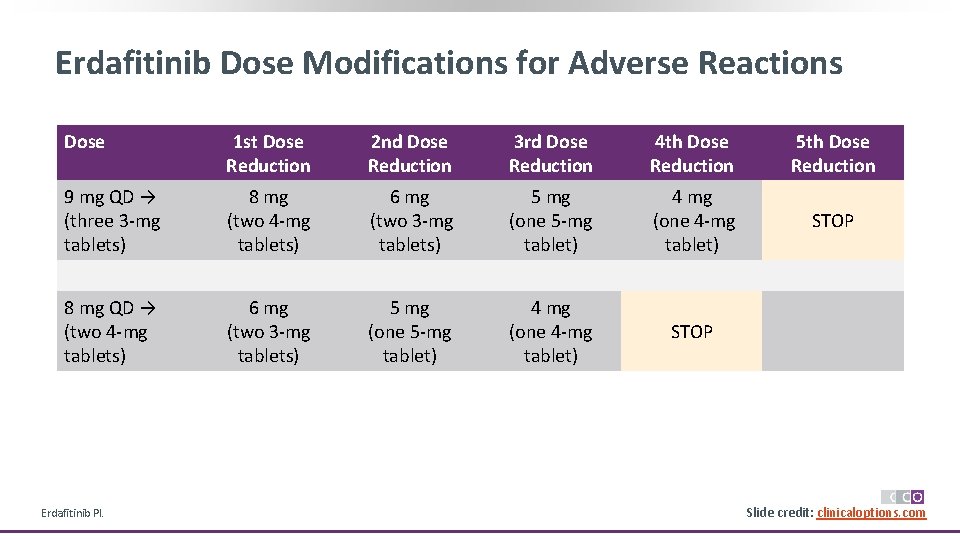
Erdafitinib Dose Modifications for Adverse Reactions Dose 1 st Dose Reduction 2 nd Dose Reduction 3 rd Dose Reduction 4 th Dose Reduction 5 th Dose Reduction 9 mg QD → (three 3 -mg tablets) 8 mg (two 4 -mg tablets) 6 mg (two 3 -mg tablets) 5 mg (one 5 -mg tablet) 4 mg (one 4 -mg tablet) STOP 8 mg QD → (two 4 -mg tablets) 6 mg (two 3 -mg tablets) 5 mg (one 5 -mg tablet) 4 mg (one 4 -mg tablet) STOP Erdafitinib PI. Slide credit: clinicaloptions. com
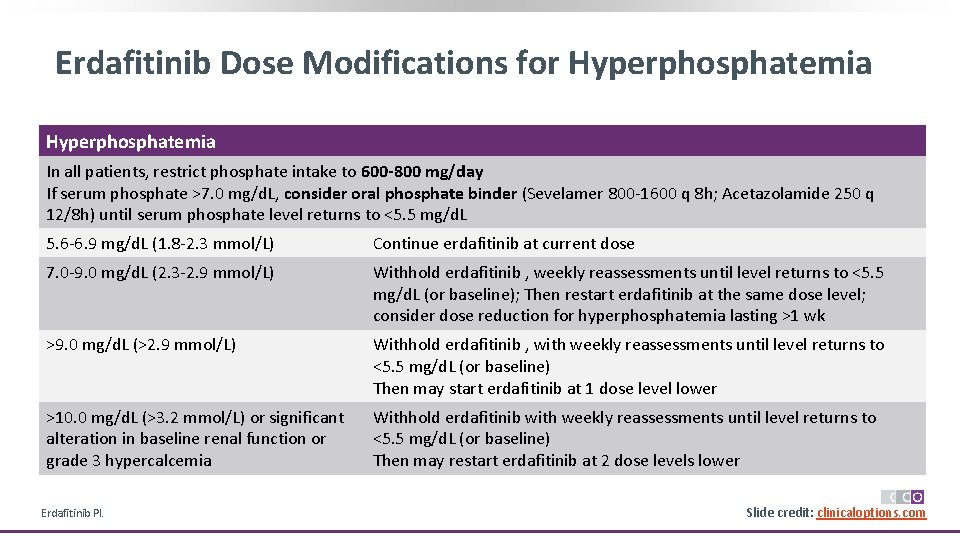
Erdafitinib Dose Modifications for Hyperphosphatemia In all patients, restrict phosphate intake to 600 -800 mg/day If serum phosphate >7. 0 mg/d. L, consider oral phosphate binder (Sevelamer 800 -1600 q 8 h; Acetazolamide 250 q 12/8 h) until serum phosphate level returns to <5. 5 mg/d. L 5. 6 -6. 9 mg/d. L (1. 8 -2. 3 mmol/L) Continue erdafitinib at current dose 7. 0 -9. 0 mg/d. L (2. 3 -2. 9 mmol/L) Withhold erdafitinib , weekly reassessments until level returns to <5. 5 mg/d. L (or baseline); Then restart erdafitinib at the same dose level; consider dose reduction for hyperphosphatemia lasting >1 wk >9. 0 mg/d. L (>2. 9 mmol/L) Withhold erdafitinib , with weekly reassessments until level returns to <5. 5 mg/d. L (or baseline) Then may start erdafitinib at 1 dose level lower >10. 0 mg/d. L (>3. 2 mmol/L) or significant alteration in baseline renal function or grade 3 hypercalcemia Withhold erdafitinib with weekly reassessments until level returns to <5. 5 mg/d. L (or baseline) Then may restart erdafitinib at 2 dose levels lower Erdafitinib PI. Slide credit: clinicaloptions. com
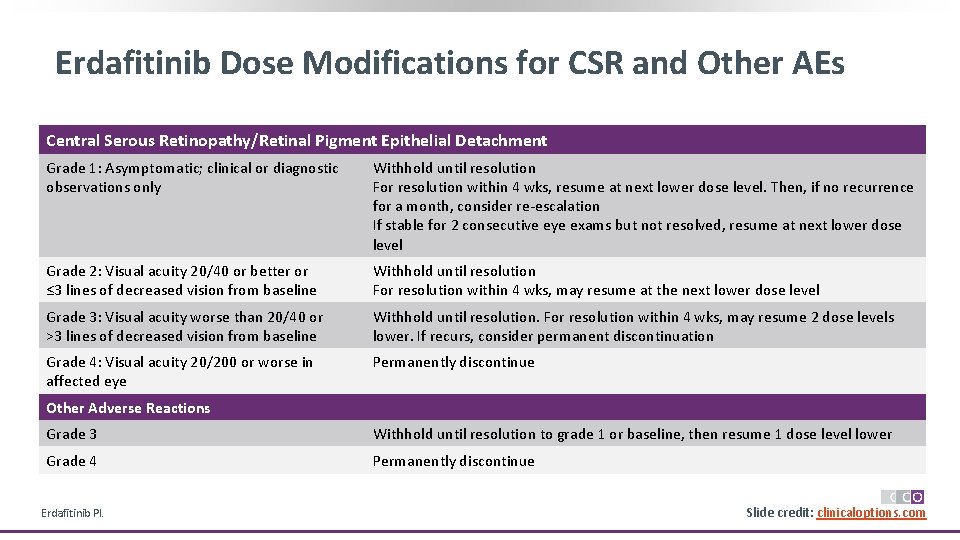
Erdafitinib Dose Modifications for CSR and Other AEs Central Serous Retinopathy/Retinal Pigment Epithelial Detachment Grade 1: Asymptomatic; clinical or diagnostic observations only Withhold until resolution For resolution within 4 wks, resume at next lower dose level. Then, if no recurrence for a month, consider re-escalation If stable for 2 consecutive eye exams but not resolved, resume at next lower dose level Grade 2: Visual acuity 20/40 or better or ≤ 3 lines of decreased vision from baseline Withhold until resolution For resolution within 4 wks, may resume at the next lower dose level Grade 3: Visual acuity worse than 20/40 or >3 lines of decreased vision from baseline Withhold until resolution. For resolution within 4 wks, may resume 2 dose levels lower. If recurs, consider permanent discontinuation Grade 4: Visual acuity 20/200 or worse in affected eye Permanently discontinue Other Adverse Reactions Grade 3 Withhold until resolution to grade 1 or baseline, then resume 1 dose level lower Grade 4 Permanently discontinue Erdafitinib PI. Slide credit: clinicaloptions. com
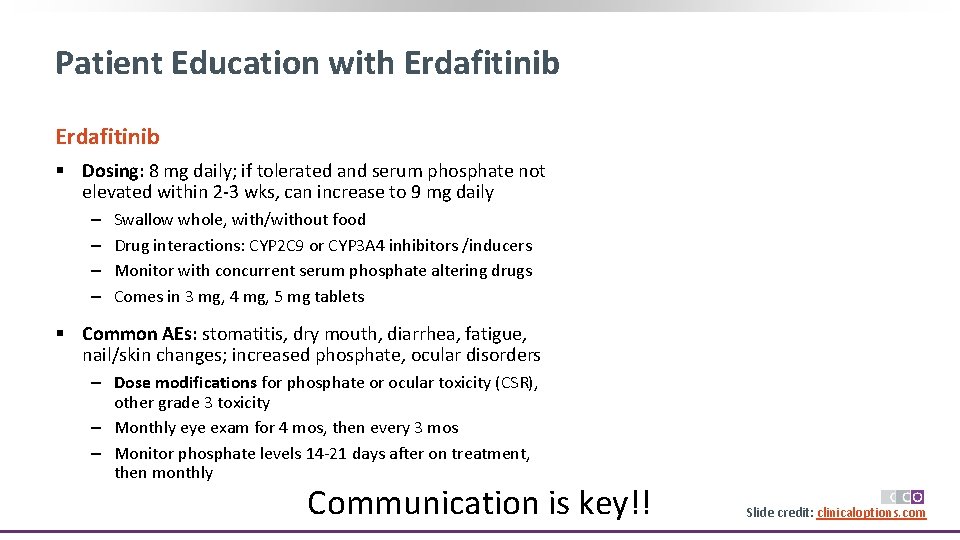
Patient Education with Erdafitinib § Dosing: 8 mg daily; if tolerated and serum phosphate not elevated within 2 -3 wks, can increase to 9 mg daily ‒ ‒ Swallow whole, with/without food Drug interactions: CYP 2 C 9 or CYP 3 A 4 inhibitors /inducers Monitor with concurrent serum phosphate altering drugs Comes in 3 mg, 4 mg, 5 mg tablets § Common AEs: stomatitis, dry mouth, diarrhea, fatigue, nail/skin changes; increased phosphate, ocular disorders ‒ Dose modifications for phosphate or ocular toxicity (CSR), other grade 3 toxicity ‒ Monthly eye exam for 4 mos, then every 3 mos ‒ Monitor phosphate levels 14 -21 days after on treatment, then monthly Communication is key!! Slide credit: clinicaloptions. com
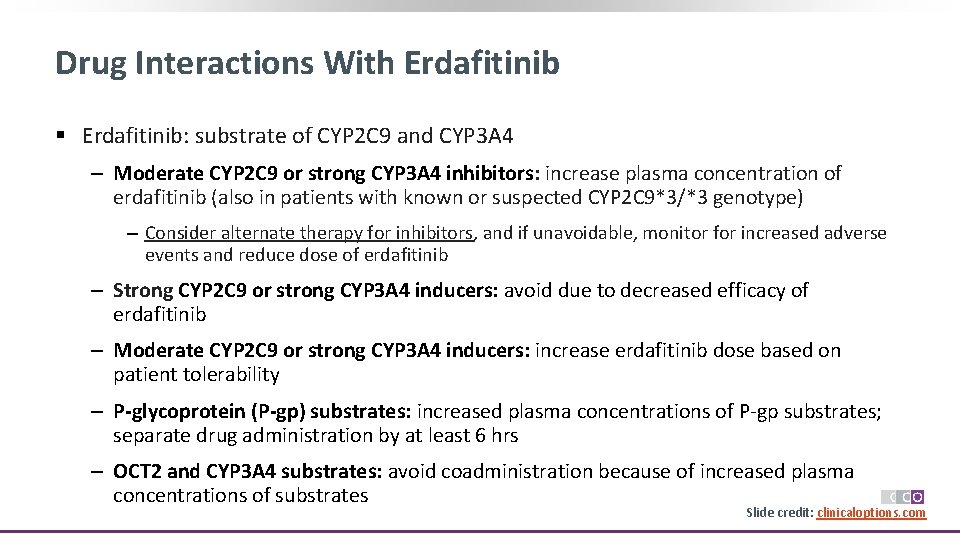
Drug Interactions With Erdafitinib § Erdafitinib: substrate of CYP 2 C 9 and CYP 3 A 4 ‒ Moderate CYP 2 C 9 or strong CYP 3 A 4 inhibitors: increase plasma concentration of erdafitinib (also in patients with known or suspected CYP 2 C 9*3/*3 genotype) ‒ Consider alternate therapy for inhibitors, and if unavoidable, monitor for increased adverse events and reduce dose of erdafitinib ‒ Strong CYP 2 C 9 or strong CYP 3 A 4 inducers: avoid due to decreased efficacy of erdafitinib ‒ Moderate CYP 2 C 9 or strong CYP 3 A 4 inducers: increase erdafitinib dose based on patient tolerability ‒ P-glycoprotein (P-gp) substrates: increased plasma concentrations of P-gp substrates; separate drug administration by at least 6 hrs ‒ OCT 2 and CYP 3 A 4 substrates: avoid coadministration because of increased plasma concentrations of substrates Slide credit: clinicaloptions. com
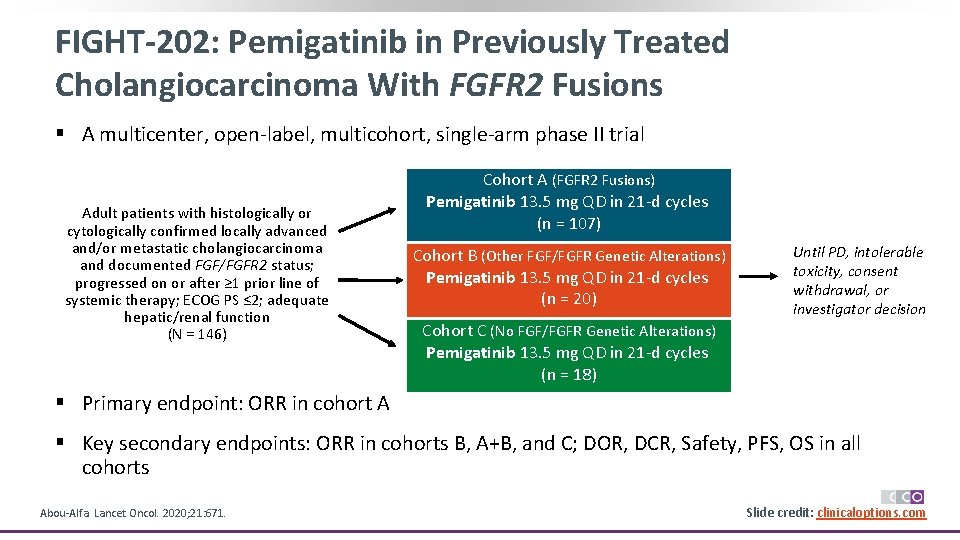
FIGHT-202: Pemigatinib in Previously Treated Cholangiocarcinoma With FGFR 2 Fusions § A multicenter, open-label, multicohort, single-arm phase II trial Adult patients with histologically or cytologically confirmed locally advanced and/or metastatic cholangiocarcinoma and documented FGF/FGFR 2 status; progressed on or after ≥ 1 prior line of systemic therapy; ECOG PS ≤ 2; adequate hepatic/renal function (N = 146) Cohort A (FGFR 2 Fusions) Pemigatinib 13. 5 mg QD in 21 -d cycles (n = 107) Cohort B (Other FGF/FGFR Genetic Alterations) Pemigatinib 13. 5 mg QD in 21 -d cycles (n = 20) Until PD, intolerable toxicity, consent withdrawal, or investigator decision Cohort C (No FGF/FGFR Genetic Alterations) Pemigatinib 13. 5 mg QD in 21 -d cycles (n = 18) § Primary endpoint: ORR in cohort A § Key secondary endpoints: ORR in cohorts B, A+B, and C; DOR, DCR, Safety, PFS, OS in all cohorts Abou-Alfa. Lancet Oncol. 2020; 21: 671. Slide credit: clinicaloptions. com
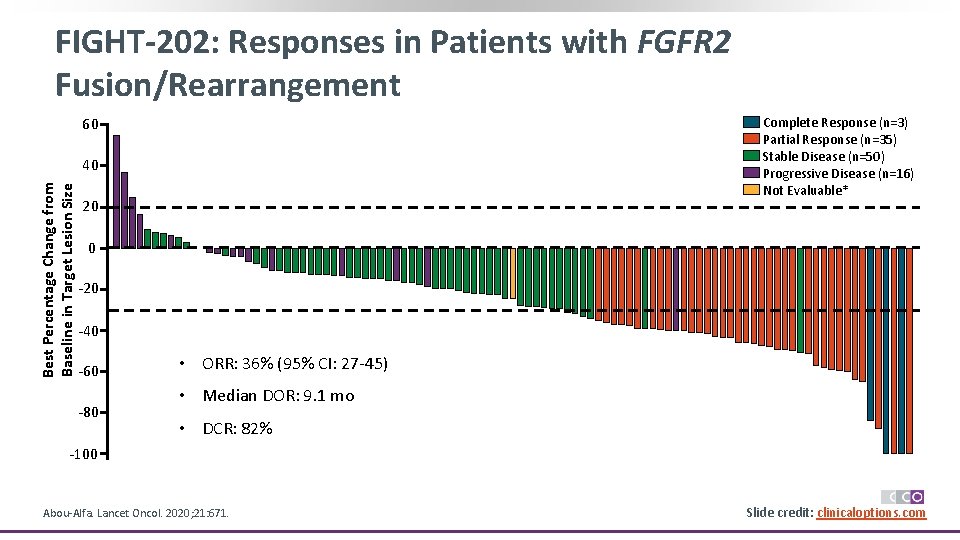
FIGHT-202: Responses in Patients with FGFR 2 Fusion/Rearrangement 60 Complete Response (n=3) Partial Response (n=35) Stable Disease (n=50) Progressive Disease (n=16) Not Evaluable* Best Percentage Change from Baseline in Target Lesion Size 40 20 0 -20 -40 -60 -80 • ORR: 36% (95% CI: 27 -45) • Median DOR: 9. 1 mo • DCR: 82% -100 Abou-Alfa. Lancet Oncol. 2020; 21: 671. Slide credit: clinicaloptions. com
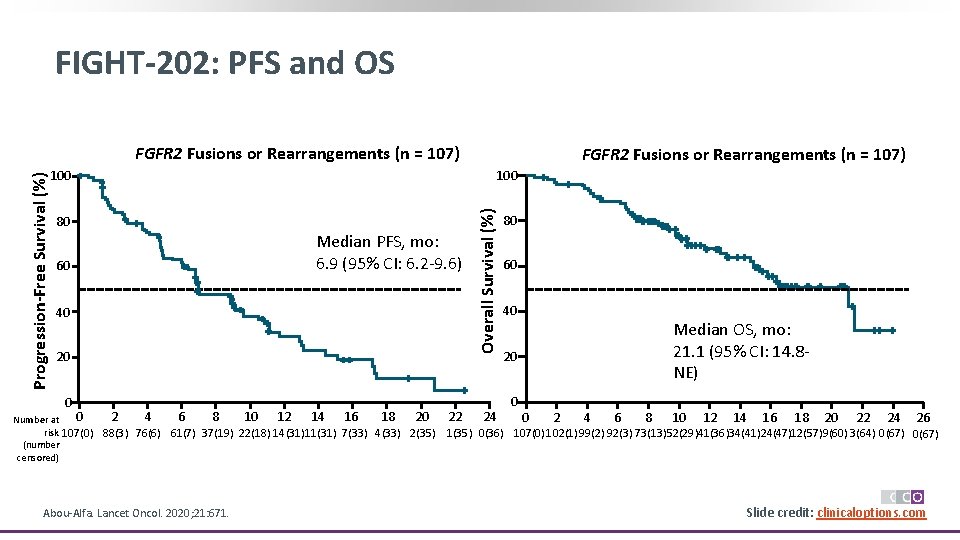
FIGHT-202: PFS and OS 100 80 60 Median PFS, mo: 6. 9 (95% CI: 6. 2 -9. 6) 40 20 0 0 2 4 6 8 10 12 14 16 18 20 Number at risk 107(0) 88(3) 76(6) 61(7) 37(19) 22(18) 14(31)11(31) 7(33) 4(33) 2(35) (number censored) Abou-Alfa. Lancet Oncol. 2020; 21: 671. FGFR 2 Fusions or Rearrangements (n = 107) 22 Overall Survival (%) Progression-Free Survival (%) FGFR 2 Fusions or Rearrangements (n = 107) 24 80 60 40 Median OS, mo: 21. 1 (95% CI: 14. 8 NE) 20 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 1(35) 0(36) 107(0) 102(1)99(2) 92(3) 73(13)52(29)41(36)34(41)24(47)12(57)9(60) 3(64) 0(67) Slide credit: clinicaloptions. com
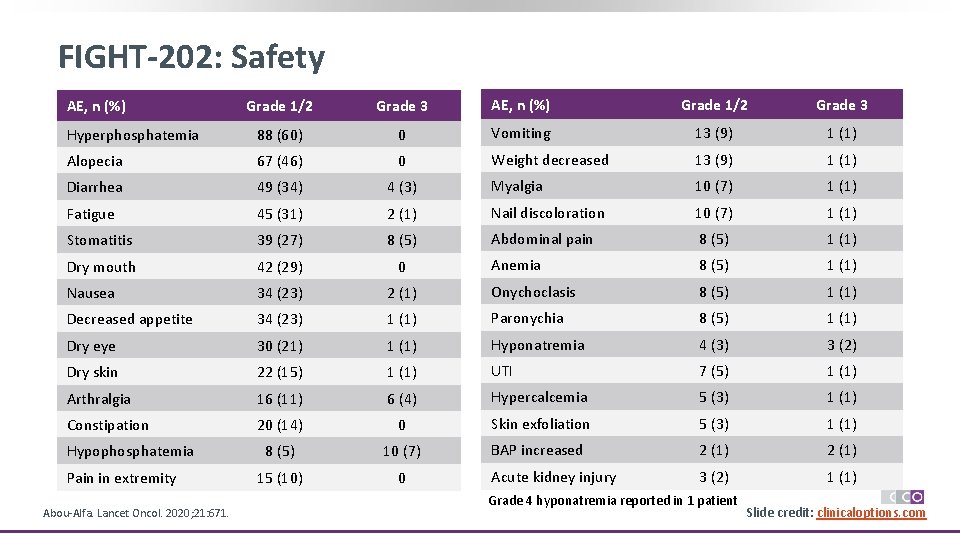
FIGHT-202: Safety Grade 1/2 Grade 3 AE, n (%) Grade 1/2 Grade 3 Hyperphosphatemia 88 (60) 0 Vomiting 13 (9) 1 (1) Alopecia 67 (46) 0 Weight decreased 13 (9) 1 (1) Diarrhea 49 (34) 4 (3) Myalgia 10 (7) 1 (1) Fatigue 45 (31) 2 (1) Nail discoloration 10 (7) 1 (1) Stomatitis 39 (27) 8 (5) Abdominal pain 8 (5) 1 (1) Dry mouth 42 (29) 0 Anemia 8 (5) 1 (1) Nausea 34 (23) 2 (1) Onychoclasis 8 (5) 1 (1) Decreased appetite 34 (23) 1 (1) Paronychia 8 (5) 1 (1) Dry eye 30 (21) 1 (1) Hyponatremia 4 (3) 3 (2) Dry skin 22 (15) 1 (1) UTI 7 (5) 1 (1) Arthralgia 16 (11) 6 (4) Hypercalcemia 5 (3) 1 (1) Constipation 20 (14) 0 Skin exfoliation 5 (3) 1 (1) 8 (5) 10 (7) BAP increased 2 (1) 15 (10) 0 Acute kidney injury 3 (2) 1 (1) AE, n (%) Hypophosphatemia Pain in extremity Abou-Alfa. Lancet Oncol. 2020; 21: 671. Grade 4 hyponatremia reported in 1 patient Slide credit: clinicaloptions. com
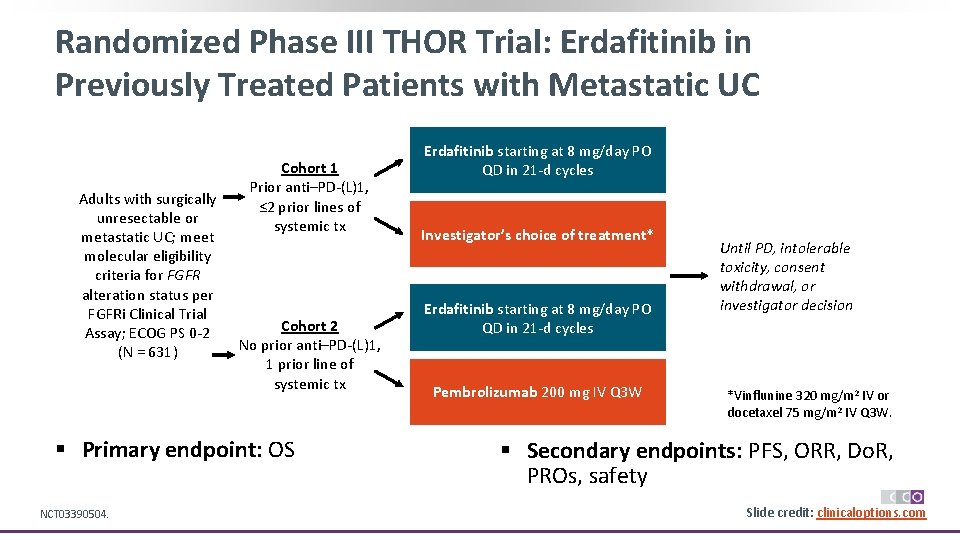
Randomized Phase III THOR Trial: Erdafitinib in Previously Treated Patients with Metastatic UC Adults with surgically unresectable or metastatic UC; meet molecular eligibility criteria for FGFR alteration status per FGFRi Clinical Trial Assay; ECOG PS 0 -2 (N = 631) Cohort 1 Prior anti–PD-(L)1, ≤ 2 prior lines of systemic tx Cohort 2 No prior anti–PD-(L)1, 1 prior line of systemic tx § Primary endpoint: OS NCT 03390504. Erdafitinib starting at 8 mg/day PO QD in 21 -d cycles Investigator’s choice of treatment* Erdafitinib starting at 8 mg/day PO QD in 21 -d cycles Pembrolizumab 200 mg IV Q 3 W Until PD, intolerable toxicity, consent withdrawal, or investigator decision *Vinflunine 320 mg/m 2 IV or docetaxel 75 mg/m 2 IV Q 3 W. § Secondary endpoints: PFS, ORR, Do. R, PROs, safety Slide credit: clinicaloptions. com
Areas of Clinical Research for FGFR Therapy in the Near Future § Combinations with other strategies § Moving to earlier settings § Development of assays optimised for the use of ct. DNA § This would improve time-to-detection of driver alterations thus enabling the rapid identification of patients for targeted therapies and the real-time detection of acquired mutations Figure not available § Knowledge advancements on the role of tumor-extrinsic mechanisms, including whether, when, and how the tumor and/or immune microenvironment might contribute to acquired resistance Krook. Br J Cancer. 2021; 124: 880. Slide credit: clinicaloptions. com

Phase I/II NORSE Trial: Erdafitinib + Cetrelimab in Previously Treated Metastatic UC DL 1 Erdafitinib 6 mg PO QD + Cetrelimab 240 mg IV Q 2 W Adults with metastatic or locally advanced UC w/FGFR alteration; progressed on or after ≥ 1 prior line of systemic therapy; ECOG PS ≤ 2 (Estimated N = 160)1, 2 DL 2 A Erdafitinib 8 mg PO QD (no uptitration) + Cetrelimab 240 mg IV Q 2 W DL 2 B Erdafitinib 8 mg PO QD (uptitration to 9 mg) + Cetrelimab 240 mg IV Q 2 W (initiated at cycle 2 day 1) Until PD, intolerable toxicity, consent withdrawal, or investigator decision DL 2 Erdafitinib 8 mg PO QD (uptitration to 9 mg) + Cetrelimab 240 mg IV Q 2 W § Primary endpoint: DLTs, safety (phase Ib); ORR and § safety (phase II) § Secondary endpoints: PK, safety (Phase Ib); PK, safety, Do. R, time to response, PFS, OS (phase II) 1. Siefker-Radtke. ESMO 2020. Abstr 752 P 2. NCT 03473743. 3. Moreno. ASCO 2020. Abstr 3055. Results: In 22 treated patients; confirmed ORR 55% in evaluable patients with 100% DCR; TEAEs and TRAEs observed in most patients 3 Slide credit: clinicaloptions. com
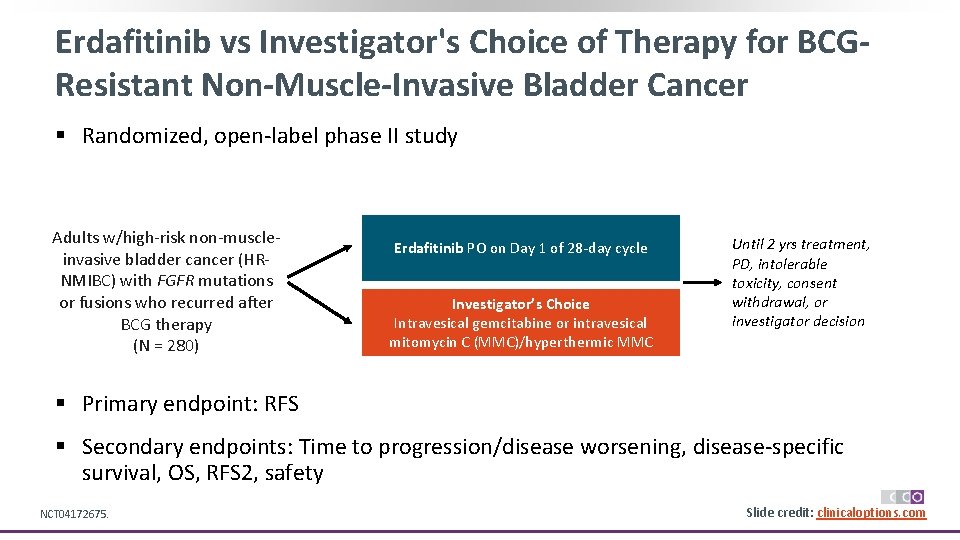
Erdafitinib vs Investigator's Choice of Therapy for BCGResistant Non-Muscle-Invasive Bladder Cancer § Randomized, open-label phase II study Adults w/high-risk non-muscleinvasive bladder cancer (HRNMIBC) with FGFR mutations or fusions who recurred after BCG therapy (N = 280) Erdafitinib PO on Day 1 of 28 -day cycle Investigator’s Choice Intravesical gemcitabine or intravesical mitomycin C (MMC)/hyperthermic MMC Until 2 yrs treatment, PD, intolerable toxicity, consent withdrawal, or investigator decision § Primary endpoint: RFS § Secondary endpoints: Time to progression/disease worsening, disease-specific survival, OS, RFS 2, safety NCT 04172675. Slide credit: clinicaloptions. com
Bladder Cancer and Beyond: Conclusions § Precision medicine in bladder cancer has arrived! § FGF-FGFR pathway is critical for carcinogenesis in many tumors, including bladder cancer and cholangiosarcoma ‒ Identification of patients that can receive a targeted therapy based on genetic abnormalities in FGFR is possible § Erdafitinib effective in metastatic UC (BLC-2001 Study): 40% ORR in previously treated patients with negative prognostic factors (eg, visceral disease) Slide credit: clinicaloptions. com
Go Online for More CCO Coverage of Bladder Cancer! Downloadable slideset with Clinical Express. Points on the management of bladder cancer from this program Additional slides and programs on bladder cancer clinicaloptions. com/oncology
- Slides: 46