Targeted Therapies Gynaecologische kankers ER HERs Angiogenese RANKL








![Neoadjuvant HER block: % p. CR ~ ER-status [HER-2 (+)] Combin > Herceptin > Neoadjuvant HER block: % p. CR ~ ER-status [HER-2 (+)] Combin > Herceptin >](https://slidetodoc.com/presentation_image/8bb0db1f364d7a7794b75fa8ad6147a5/image-19.jpg)



















- Slides: 69

“Targeted Therapies” Gynaecologische kankers ER HERs Angiogenese RANK-L PI 3 K, AKT, MTor FGF-R, ILGF-R (…) P. Neven GYN ONCOL UZ Leuven VVOG- Bredene - 2012

Disclosure Slide • None to be declared

‘Targeted therapies’ Gynecologische Tumoren ‘Tumors’ are “oncogen” addicted Despite the genomic & epigenomic complexities tumor regression can occur with inactivation of 1 or 2 oncogene(s) “Cancer Addiction to Oncogene” : The Achilles Heal of Cancer Weinstein IB Science 2002 Betere kennis mechanisme van kankerprogressie op cellulair en moleculair niveau moleculair-gebaseerde en geïndividualiseerde therapieën nut van therapie stijgt , onnodig nadeel daalt, en meer ‘cost-efficiënt’

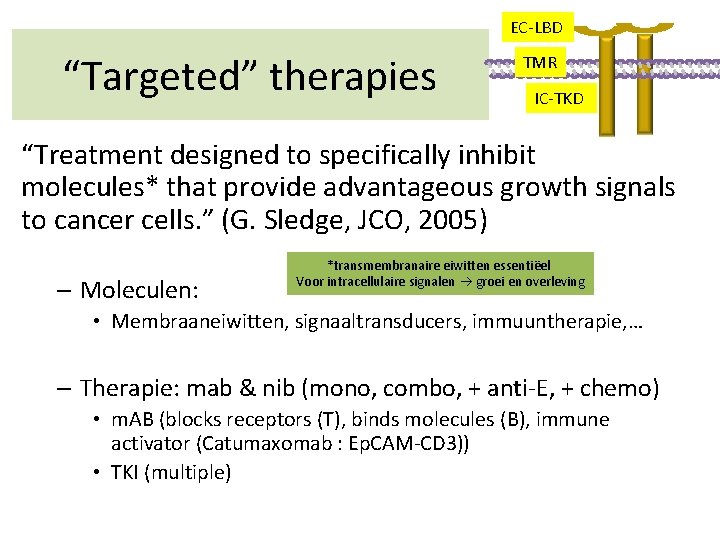
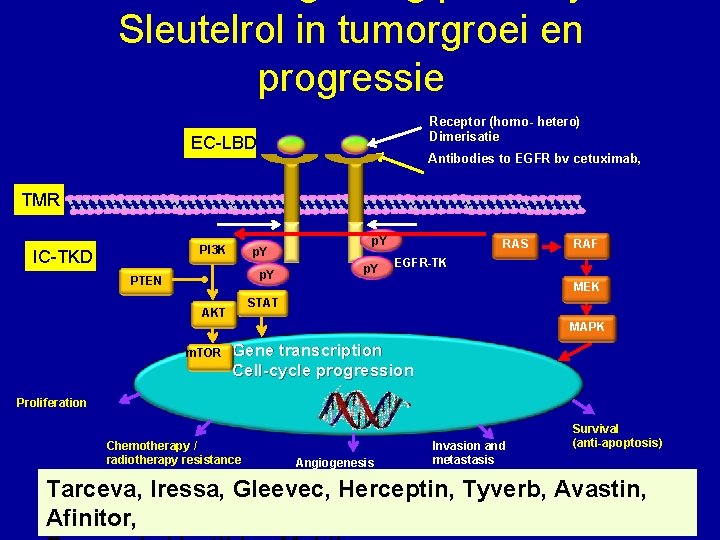
EC-LBD “Targeted” therapies TMR IC-TKD “Treatment designed to specifically inhibit molecules* that provide advantageous growth signals to cancer cells. ” (G. Sledge, JCO, 2005) – Moleculen: *transmembranaire eiwitten essentiëel Voor intracellulaire signalen groei en overleving • Membraaneiwitten, signaaltransducers, immuuntherapie, … – Therapie: mab & nib (mono, combo, + anti-E, + chemo) • m. AB (blocks receptors (T), binds molecules (B), immune activator (Catumaxomab : Ep. CAM-CD 3)) • TKI (multiple)

Sleutelrol in tumorgroei en progressie Receptor (homo- hetero) Dimerisatie Ligand EC-LBD Antibodies to EGFR bv cetuximab, TMR PI 3 K IC-TKD p. Y PTEN p. Y RAS RAF EGFR-TK MEK STAT AKT MAPK m. TOR Gene transcription Cell-cycle progression Proliferation Chemotherapy / radiotherapy resistance Angiogenesis Invasion and metastasis Survival (anti-apoptosis) Tarceva, Iressa, Gleevec, Herceptin, Tyverb, Avastin, Meyerhardt & Mayer, N Engl J Med 2005 Venook, Oncologist 2005 Afinitor,

Targets met Targeted Therapies Farmacologische inhibitie ~response • • • Steroid receptors: ER+ borst, AR+ prostaat HER 2: borst, maag ALK: NSCLC CD 20: Lymphoma Targets ~ tumorgroei, … Response ~ Target? bcr/Abl: CML c-Kit: GIST Hedgehog: Basal cell, Medulloblastoma RET: Medullair Thyroid Carcinoma b-RAF, MEK: Melanoma • EGFR: CRC, Long, H&N (ras, EGFR mutatie) • VEGF-targeted therapy: alle kankers (niet RCC) • m. TOR: Borst, Nier, … but no target so far Effect therapie ~ Aantal Mutaties (TNBC tov ER-pos, Rokers)

Overzicht presentatie Targeted Therapies • Borst & Pelvien (kliniek & toekomst) – – – ER ; BMI HER-1; HER-2; HER-3 PTEN/AKT/m. TOR en MEK-MAPK pathway VEGF PARP …

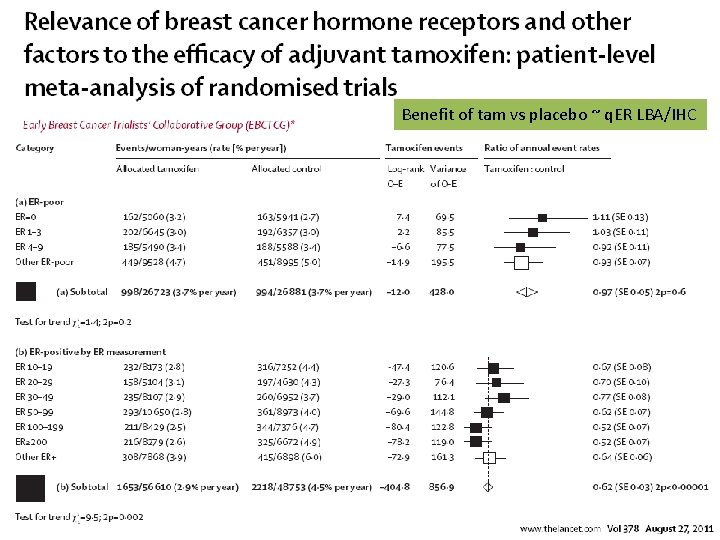
NSABP B-14 q. ER = Predict for response to TAM Low levels ‘relative resistance’ RT-PCR but also for IHC?

Benefit of tam vs placebo ~ q. ER LBA/IHC

Aromatase Inhibitors Na MENOPAUZE Target = Fat Target = Total Body Aromatisation A Reduce Estrogen • Aromatase Inhibitors E Anastrozole Letrozole Aromasin Block ER • SERMs (Tamoxifen) ER

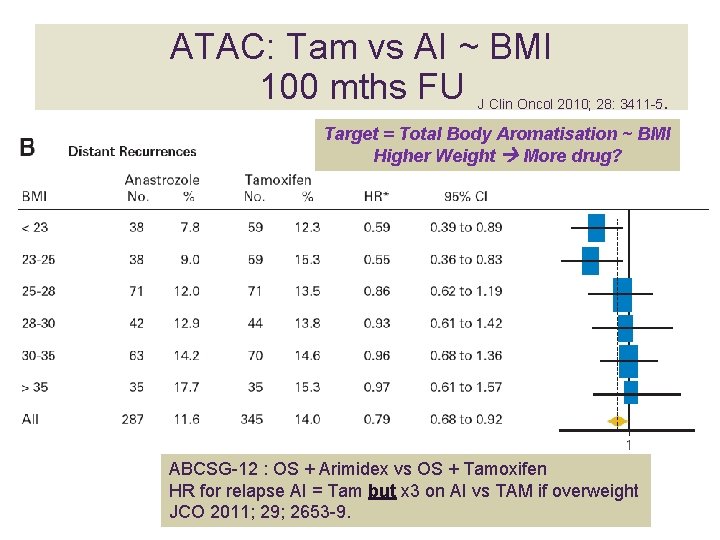
ATAC: Tam vs AI ~ BMI 100 mths FU J Clin Oncol 2010; 28: 3411 -5 . Target = Total Body Aromatisation ~ BMI Higher Weight More drug? ABCSG-12 : OS + Arimidex vs OS + Tamoxifen HR for relapse AI = Tam but x 3 on AI vs TAM if overweight JCO 2011; 29; 2653 -9.

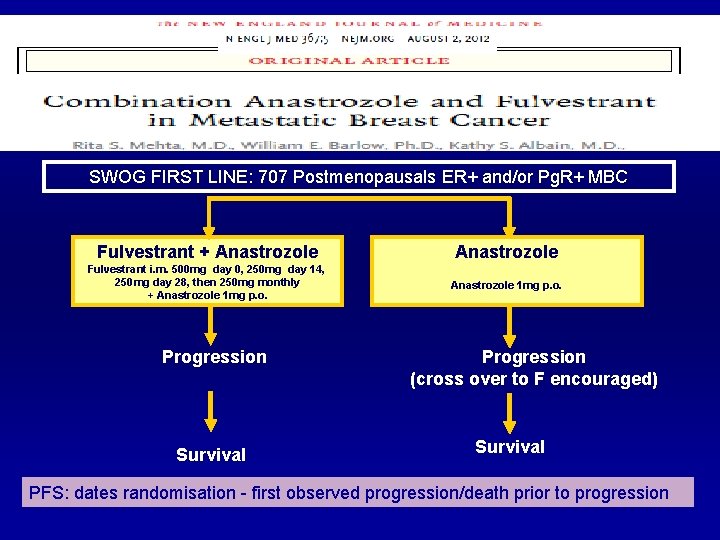
SWOG FIRST LINE: 707 Postmenopausals ER+ and/or Pg. R+ MBC Fulvestrant + Anastrozole Fulvestrant i. m. 500 mg day 0, 250 mg day 14, 250 mg day 28, then 250 mg monthly + Anastrozole 1 mg p. o. Progression Survival Progression (cross over to F encouraged) Survival PFS: dates randomisation - first observed progression/death prior to progression

+ 8 months OS-benefit

Targeted Therapies Borstkanker Target: HER-2

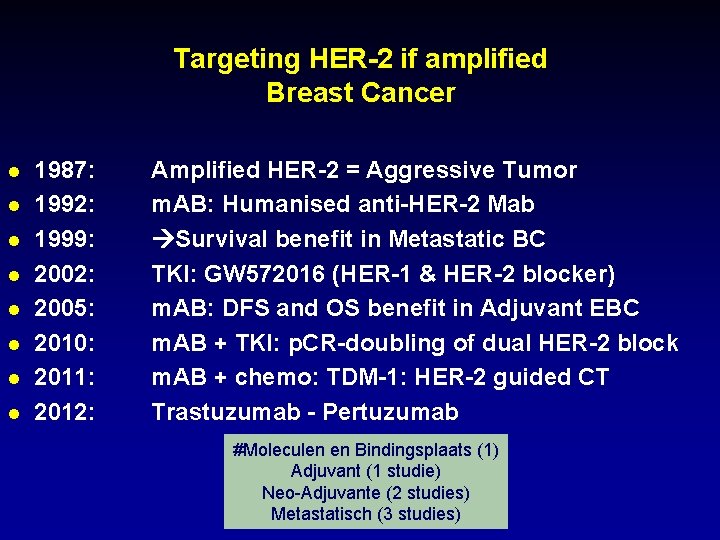
Targeting HER-2 if amplified Breast Cancer l l l l 1987: 1992: 1999: 2002: 2005: 2010: 2011: 2012: Amplified HER-2 = Aggressive Tumor m. AB: Humanised anti-HER-2 Mab Survival benefit in Metastatic BC TKI: GW 572016 (HER-1 & HER-2 blocker) m. AB: DFS and OS benefit in Adjuvant EBC m. AB + TKI: p. CR-doubling of dual HER-2 block m. AB + chemo: TDM-1: HER-2 guided CT Trastuzumab - Pertuzumab #Moleculen en Bindingsplaats (1) Adjuvant (1 studie) Neo-Adjuvante (2 studies) Metastatisch (3 studies)

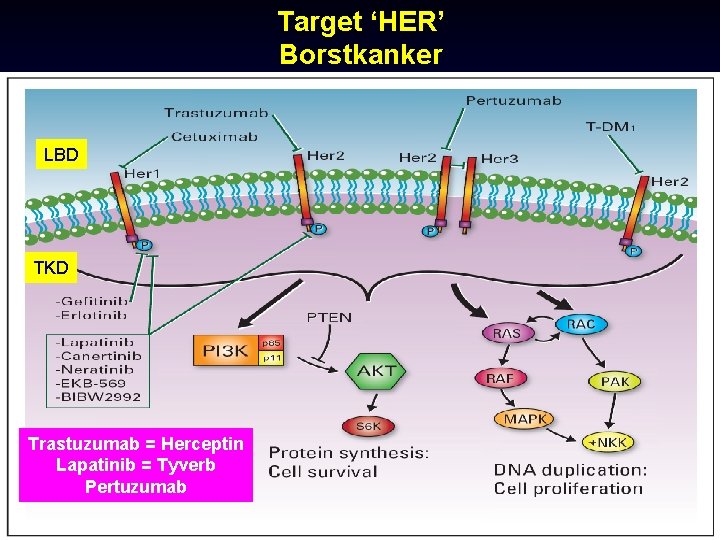
Target ‘HER’ Borstkanker LBD TKD Trastuzumab = Herceptin Lapatinib = Tyverb Pertuzumab

HER-2: Herceptin AC TH 87% D % F S 85% AC T 75% AC T H 67% AC TH N Events 1679 261 1672 134 HR=0. 48, 2 P=3 x 10 -12 Years From Randomization B 31/N 9831

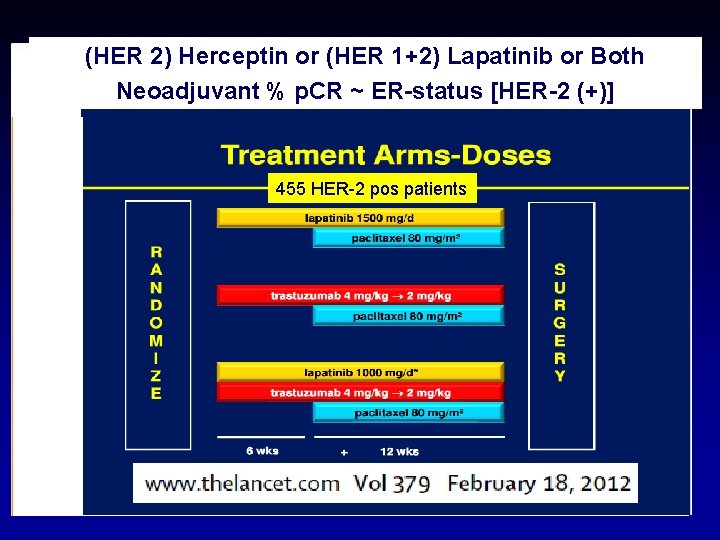
(HER 2) Herceptin or (HER 1+2) Lapatinib or Both Neoadjuvant BOTHHERCEPTIN % p. CR ~ ER-status [HER-2 (+)] 455 HER-2 pos patients
![Neoadjuvant HER block p CR ERstatus HER2 Combin Herceptin Neoadjuvant HER block: % p. CR ~ ER-status [HER-2 (+)] Combin > Herceptin >](https://slidetodoc.com/presentation_image/8bb0db1f364d7a7794b75fa8ad6147a5/image-19.jpg)
Neoadjuvant HER block: % p. CR ~ ER-status [HER-2 (+)] Combin > Herceptin > Lapatinib: Diarrhea 25% & Low Compliance ALTTO-Trial: Stop Lap mono- arm p C R %

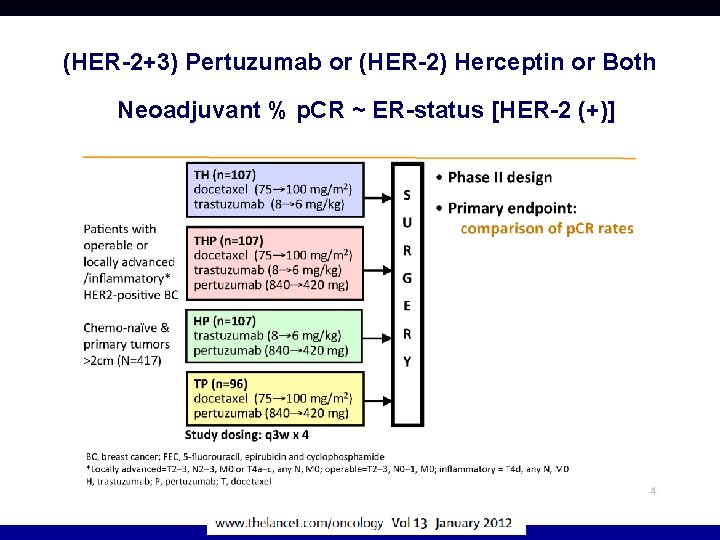
(HER-2+3) Pertuzumab or (HER-2) Herceptin or Both Neoadjuvant % p. CR ~ ER-status [HER-2 (+)] Ppp pppppp

(HER-2+3) Pertuzumab or (HER-2) Herceptin or Both Is CT needed if combined? Combinatie >Herceptin>Pertuzumab ER-Neg: No CT H+P = CT-P ▲ + chemo ▲ no chemo

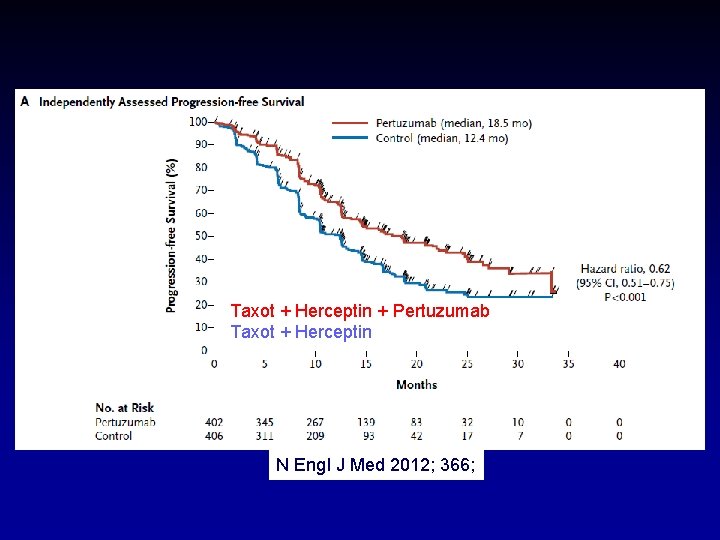
Neo-adjuv setting: “Combining Better” Herceptin + Pertuzumab >+ Lapatinib (diarrhea) Metastatic Setting 1 ste Lijn Logisch: Herceptin + Pertuzumab was toekomst. . en nu bewezen N Engl J Med 2012; 366;

Taxot + Herceptin + Pertuzumab Taxot + Herceptin N Engl J Med 2012; 366;

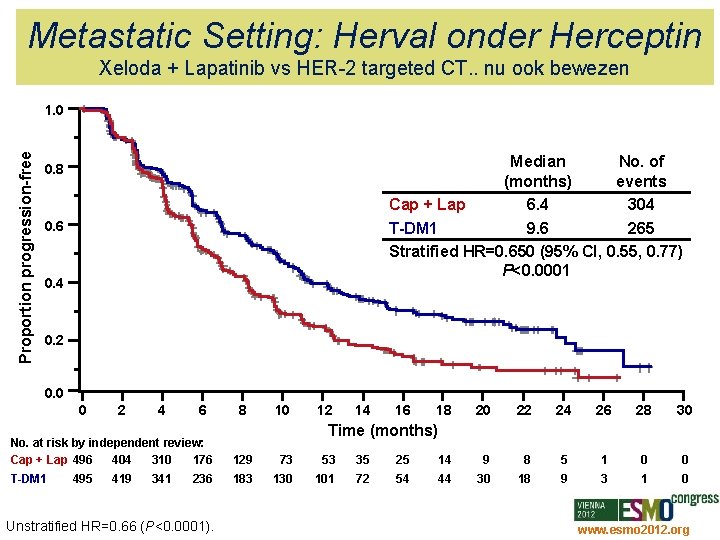
Metastatic Setting: Herval onder Herceptin Xeloda + Lapatinib vs HER-2 targeted CT. . nu ook bewezen Proportion progression-free 1. 0 Median No. of (months) events Cap + Lap 6. 4 304 T-DM 1 9. 6 265 Stratified HR=0. 650 (95% CI, 0. 55, 0. 77) P<0. 0001 0. 8 0. 6 0. 4 0. 2 0. 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 Time (months) No. at risk by independent review: Cap + Lap 496 404 310 176 129 73 53 35 25 14 9 8 5 1 0 0 T-DM 1 183 130 101 72 54 44 30 18 9 3 1 0 495 419 341 236 Unstratified HR=0. 66 (P<0. 0001). www. esmo 2012. org 24

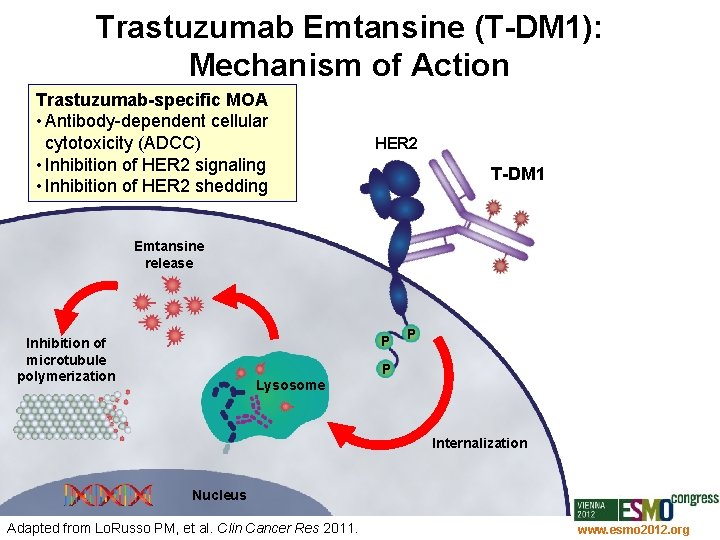
Trastuzumab Emtansine (T-DM 1): Mechanism of Action Trastuzumab-specific MOA • Antibody-dependent cellular cytotoxicity (ADCC) • Inhibition of HER 2 signaling • Inhibition of HER 2 shedding HER 2 T-DM 1 Emtansine release P Inhibition of microtubule polymerization P P Lysosome Internalization Nucleus Adapted from Lo. Russo PM, et al. Clin Cancer Res 2011. www. esmo 2012. org 25

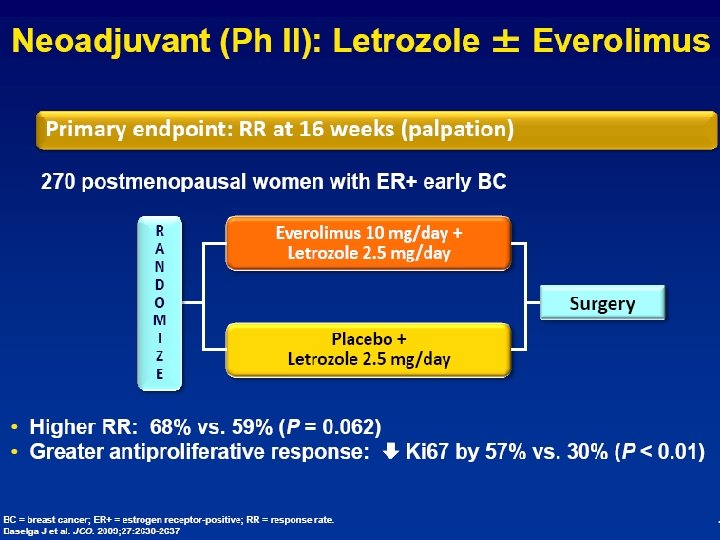
HER-2 (+): Letrozole + Lapatinib > Letrozole Wat met HER-2 (-) borstkankers? (de novo) Endocrine Resistance Behandel ook hier juiste “target”… l l l Target may change over time Outgrowth u ER-pos but ET resistant clone? Rebiopsy!!! u ER-negative (HER-2 +) clone? Downregulation of ER (promotor methylation) u Reactivate ER-expression (histone deacetylase) Enhanced peptide growth factor signalling u EGFR, HER-2, u Loss Pg. R, activation u PTEN/AKT/m. TOR or MAPK pathway u …





137 deaths 17. 2 % E + E 22. 7% E + P

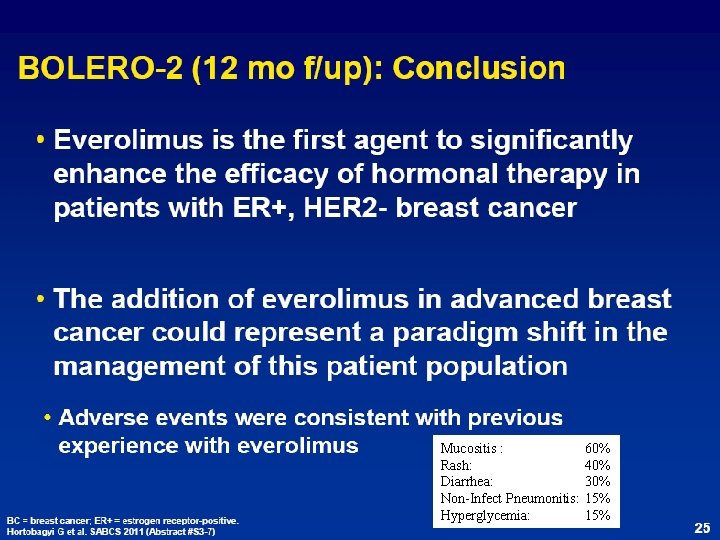
Mucositis : Rash: Diarrhea: Non-Infect Pneumonitis: Hyperglycemia: 60% 40% 30% 15%

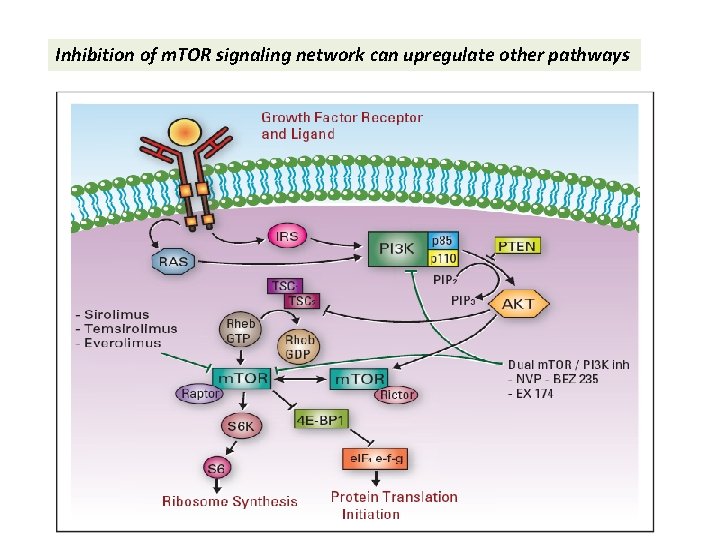
Inhibition of m. TOR signaling network can upregulate other pathways

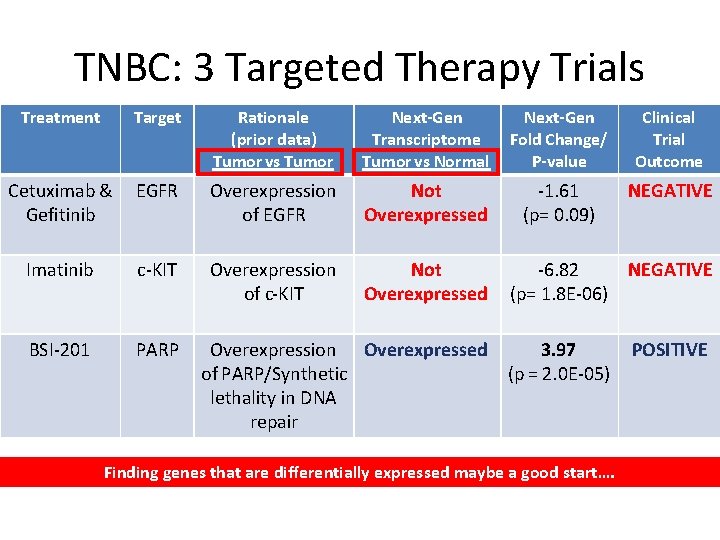
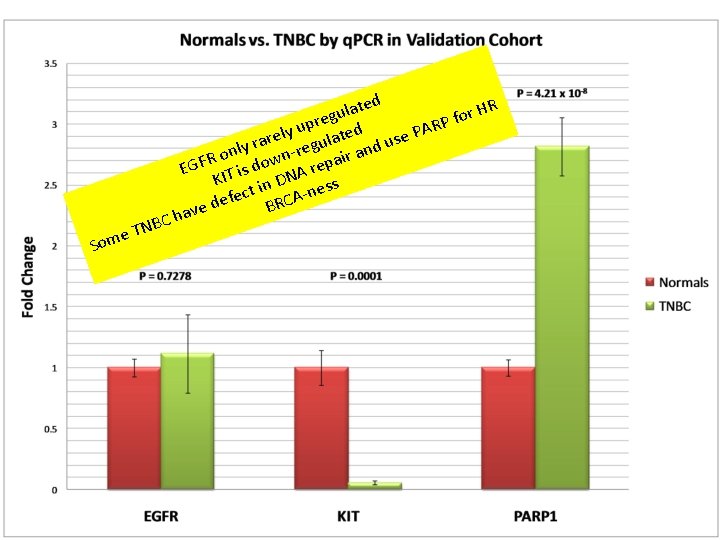
TNBC: 3 Targeted Therapy Trials Treatment Target Rationale (prior data) Tumor vs Tumor Next-Gen Transcriptome Tumor vs Normal Next-Gen Fold Change/ P-value Clinical Trial Outcome Cetuximab & Gefitinib EGFR Overexpression of EGFR Not Overexpressed -1. 61 (p= 0. 09) NEGATIVE Imatinib c-KIT Overexpression of c-KIT Not Overexpressed BSI-201 PARP -6. 82 NEGATIVE (p= 1. 8 E-06) Overexpression Overexpressed 3. 97 of PARP/Synthetic (p = 2. 0 E-05) lethality in DNA repair Finding genes that are differentially expressed maybe a good start…. POSITIVE

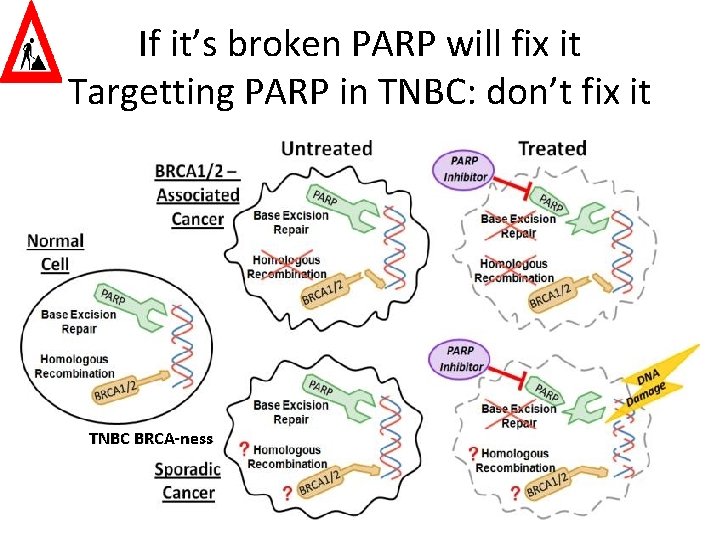
If it’s broken PARP will fix it Targetting PARP in TNBC: don’t fix it TNBC BRCA-ness

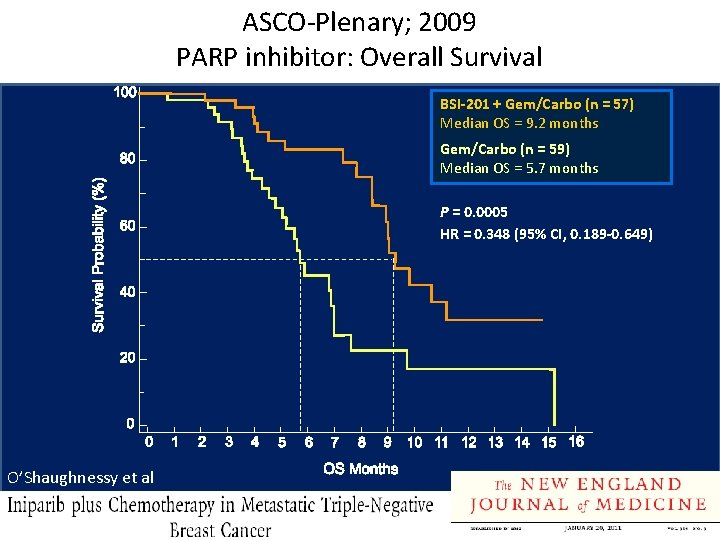
ASCO-Plenary; 2009 PARP inhibitor: Overall Survival BSI-201 + Gem/Carbo (n = 57) Median OS = 9. 2 months Gem/Carbo (n = 59) Median OS = 5. 7 months P = 0. 0005 HR = 0. 348 (95% CI, 0. 189 -0. 649) O’Shaughnessy et al

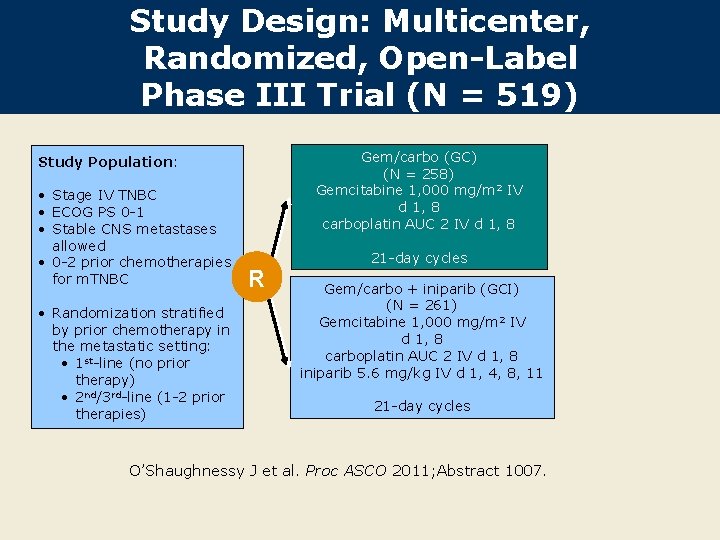
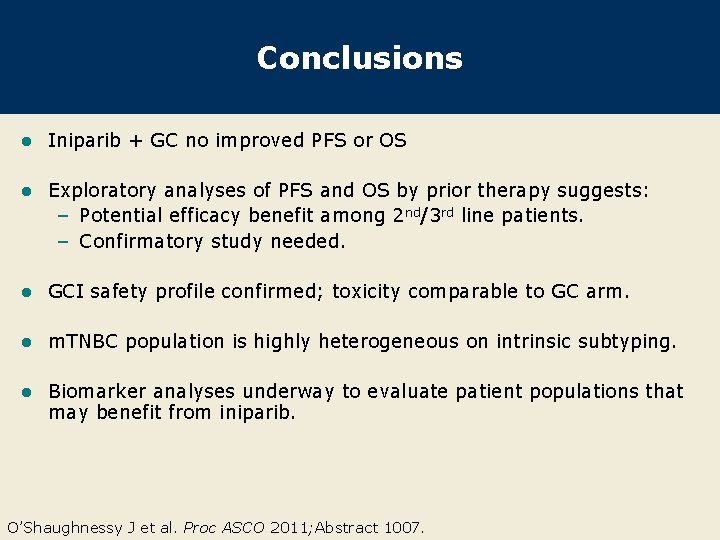
Study Design: Multicenter, Randomized, Open-Label Phase III Trial (N = 519) Gem/carbo (GC) (N = 258) Gemcitabine 1, 000 mg/m 2 IV d 1, 8 carboplatin AUC 2 IV d 1, 8 Study Population: • Stage IV TNBC • ECOG PS 0 -1 • Stable CNS metastases allowed • 0 -2 prior chemotherapies for m. TNBC • Randomization stratified by prior chemotherapy in the metastatic setting: • 1 st-line (no prior therapy) • 2 nd/3 rd-line (1 -2 prior therapies) R 21 -day cycles Gem/carbo + iniparib (GCI) (N = 261) Gemcitabine 1, 000 mg/m 2 IV d 1, 8 carboplatin AUC 2 IV d 1, 8 iniparib 5. 6 mg/kg IV d 1, 4, 8, 11 21 -day cycles O’Shaughnessy J et al. Proc ASCO 2011; Abstract 1007.

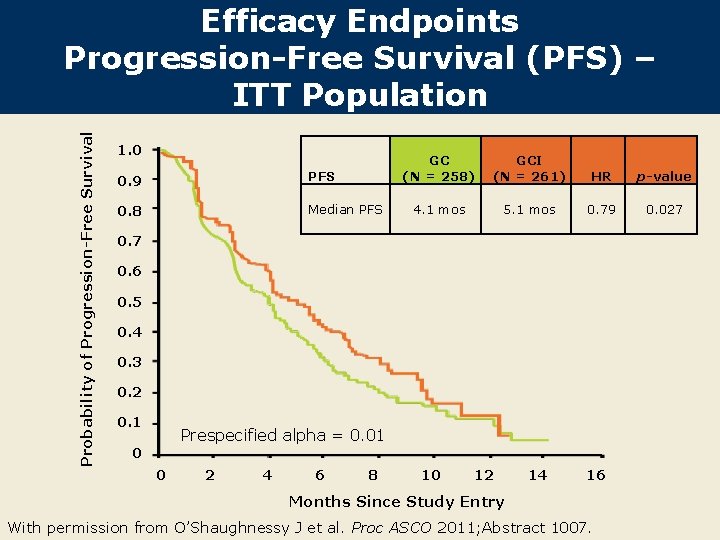
Probability of Progression-Free Survival Efficacy Endpoints Progression-Free Survival (PFS) – ITT Population 1. 0 0. 9 PFS 0. 8 Median PFS GC (N = 258) GCI (N = 261) HR p-value 4. 1 mos 5. 1 mos 0. 79 0. 027 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 Prespecified alpha = 0. 01 0 0 2 4 6 8 10 12 14 16 Months Since Study Entry With permission from O’Shaughnessy J et al. Proc ASCO 2011; Abstract 1007.

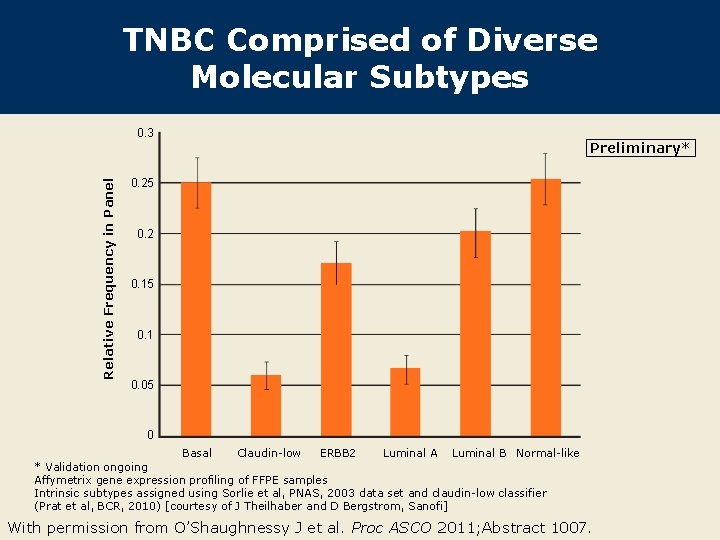
TNBC Comprised of Diverse Molecular Subtypes Relative Frequency in Panel 0. 3 Preliminary* 0. 25 0. 2 0. 15 0. 1 0. 05 0 Basal Claudin-low ERBB 2 Luminal A Luminal B Normal-like * Validation ongoing Affymetrix gene expression profiling of FFPE samples Intrinsic subtypes assigned using Sorlie et al, PNAS, 2003 data set and claudin-low classifier (Prat et al, BCR, 2010) [courtesy of J Theilhaber and D Bergstrom, Sanofi] With permission from O’Shaughnessy J et al. Proc ASCO 2011; Abstract 1007.

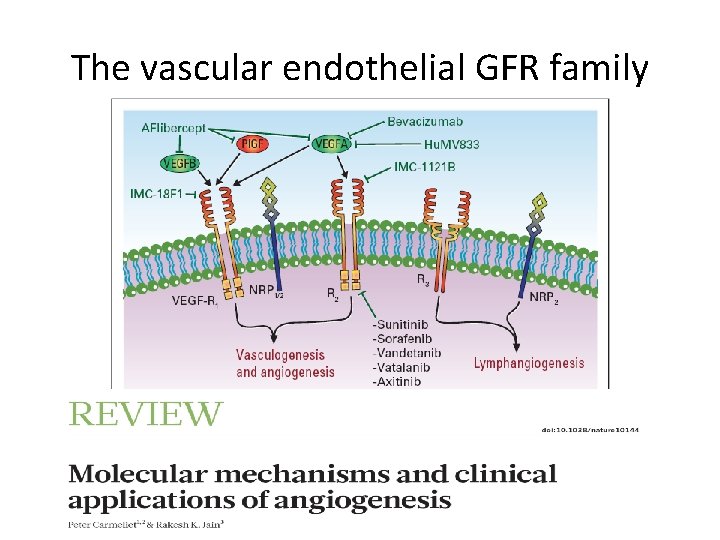
The vascular endothelial GFR family

Targets in pelviene tumoren • ER, Pg. R, k. RAS, PTEN, P 53, HER-1 &2 – Anti-E – Mo. Ab: Encouraging results only… • VEGF (DFS) & EGFR & HER-2 & FGF-FGFR • Ep. CAM, CA 125, MUC 1, Folate Receptor, CTLA-4 • Ovarium>Cervix>Endometrium – Catumaxomab (ascites) NIB’s


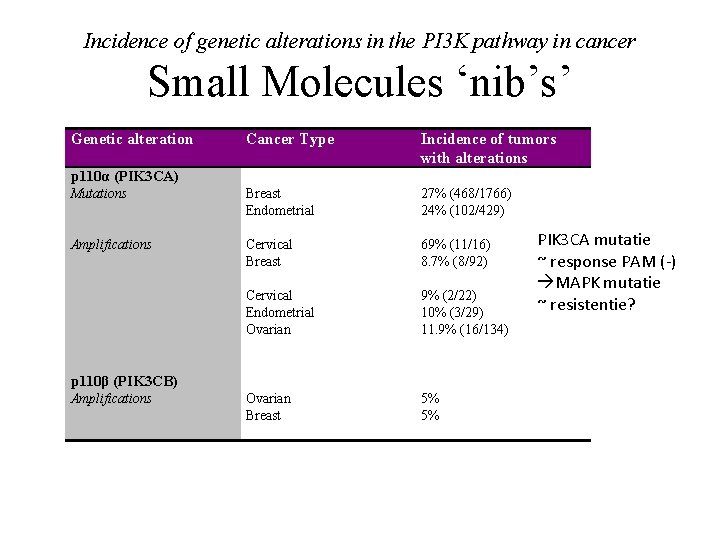
Incidence of genetic alterations in the PI 3 K pathway in cancer Small Molecules ‘nib’s’ Genetic alteration Cancer Type Incidence of tumors with alterations p 110α (PIK 3 CA) Breast Endometrial Cervical Breast Cervical Endometrial Ovarian 27% (468/1766) 24% (102/429) 69% (11/16) 8. 7% (8/92) 9% (2/22) 10% (3/29) 11. 9% (16/134) Amplifications Ovarian Breast 5% 5% Mutations Amplifications p 110β (PIK 3 CB) PIK 3 CA mutatie ~ response PAM (-) MAPK mutatie ~ resistentie?

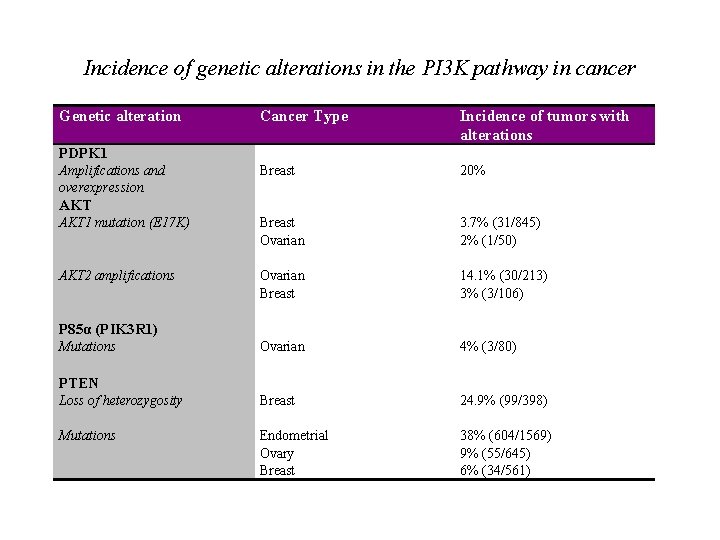
Incidence of genetic alterations in the PI 3 K pathway in cancer Genetic alteration Cancer Type Incidence of tumors with alterations PDPK 1 Breast 20% Breast Ovarian Breast Endometrial Ovary Breast 3. 7% (31/845) 2% (1/50) 14. 1% (30/213) 3% (3/106) 4% (3/80) 24. 9% (99/398) 38% (604/1569) 9% (55/645) 6% (34/561) Amplifications and overexpression AKT 1 mutation (E 17 K) AKT 2 amplifications P 85α (PIK 3 R 1) Mutations PTEN Loss of heterozygosity Mutations

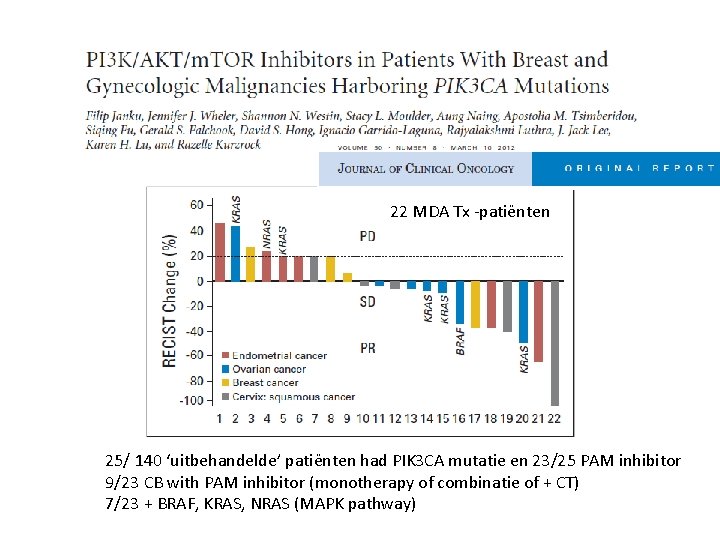
22 MDA Tx -patiënten 25/ 140 ‘uitbehandelde’ patiënten had PIK 3 CA mutatie en 23/25 PAM inhibitor 9/23 CB with PAM inhibitor (monotherapy of combinatie of + CT) 7/23 + BRAF, KRAS, NRAS (MAPK pathway)

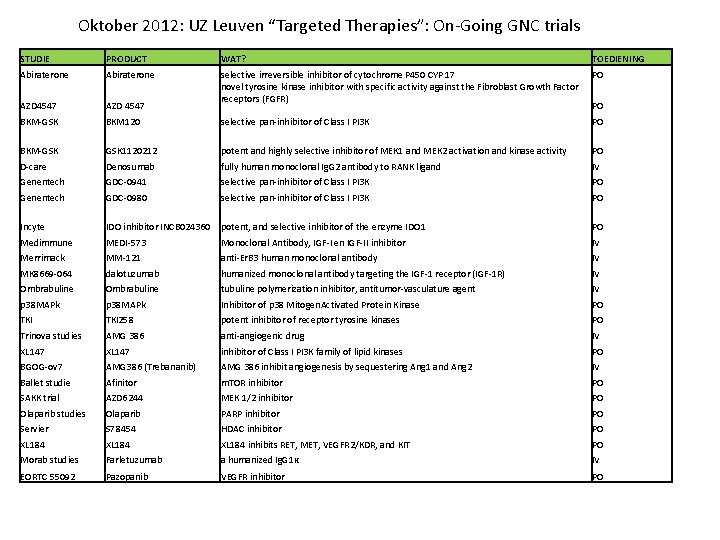
Oktober 2012: UZ Leuven “Targeted Therapies”: On-Going GNC trials STUDIE PRODUCT WAT? TOEDIENING Abiraterone PO AZD 4547 selective irreversible inhibitor of cytochrome P 450 CYP 17 novel tyrosine kinase inhibitor with specific activity against the Fibroblast Growth Factor receptors (FGFR) BKM-GSK BKM 120 selective pan-inhibitor of Class I PI 3 K PO BKM-GSK 1120212 potent and highly selective inhibitor of MEK 1 and MEK 2 activation and kinase activity PO D-care Denosumab fully human monoclonal Ig. G 2 antibody to RANK ligand IV Genentech GDC-0941 selective pan-inhibitor of Class I PI 3 K PO Genentech GDC-0980 selective pan-inhibitor of Class I PI 3 K PO Incyte IDO inhibitor INCB 024360 potent, and selective inhibitor of the enzyme IDO 1 PO Medimmune MEDI-573 Monoclonal Antibody, IGF-I en IGF-II inhibitor IV Merrimack MM-121 anti-Er. B 3 human monoclonal antibody IV MK 8669 -064 dalotuzumab humanized monoclonal antibody targeting the IGF-1 receptor (IGF-1 R) IV Ombrabuline tubuline polymerization inhibitor, antitumor-vasculature agent IV p 38 MAPk Inhibitor of p 38 Mitogen. Activated Protein Kinase PO TKI 258 potent inhibitor of receptor tyrosine kinases PO Trinova studies AMG 386 anti-angiogenic drug IV XL 147 inhibitor of Class I PI 3 K family of lipid kinases PO BGOG-ov 7 AMG 386 (Trebananib) AMG 386 inhibit angiogenesis by sequestering Ang 1 and Ang 2 IV Ballet studie Afinitor m. TOR inhibitor PO SAKK trial AZD 6244 MEK 1/2 inhibitor PO Olaparib studies Olaparib PARP inhibitor PO Servier S 78454 HDAC inhibitor PO XL 184 inhibits RET, MET, VEGFR 2/KDR, and KIT PO Morab studies Farletuzumab a humanized Ig. G 1κ IV EORTC 55092 Pazopanib VEGFR inhibitor PO PO

Take Home Message “Targeted Therapies” ------------------------------Gynaecologische kankers ------------------------------Toekomst van Therapie bij Borst en Pelviene Tumoren = Combinaties van Targeted Agents … zonder chemo…

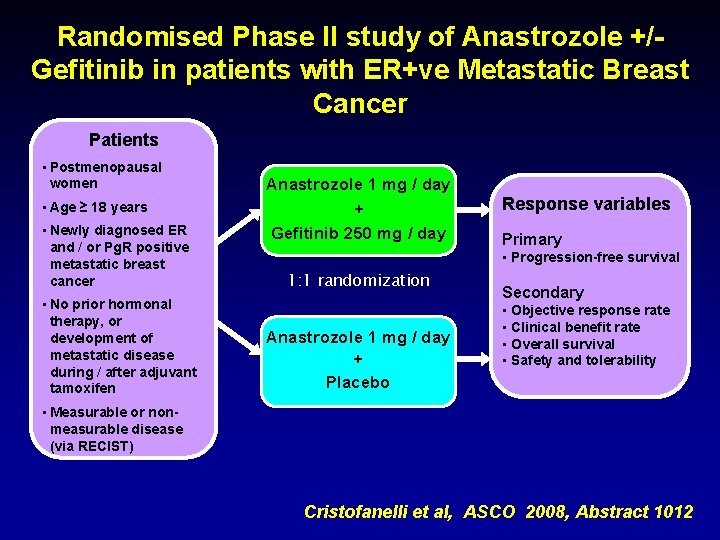
Randomised Phase II study of Anastrozole +/Gefitinib in patients with ER+ve Metastatic Breast Cancer Patients • Postmenopausal women • Age ≥ 18 years • Newly diagnosed ER and / or Pg. R positive metastatic breast cancer Anastrozole 1 mg / day + Gefitinib 250 mg / day Primary • Progression-free survival 1: 1 randomization • No prior hormonal therapy, or development of metastatic disease during / after adjuvant tamoxifen Response variables Anastrozole 1 mg / day + Placebo Secondary • Objective response rate • Clinical benefit rate • Overall survival • Safety and tolerability • Measurable or non- measurable disease (via RECIST) Cristofanelli et al, ASCO 2008, Abstract 1012

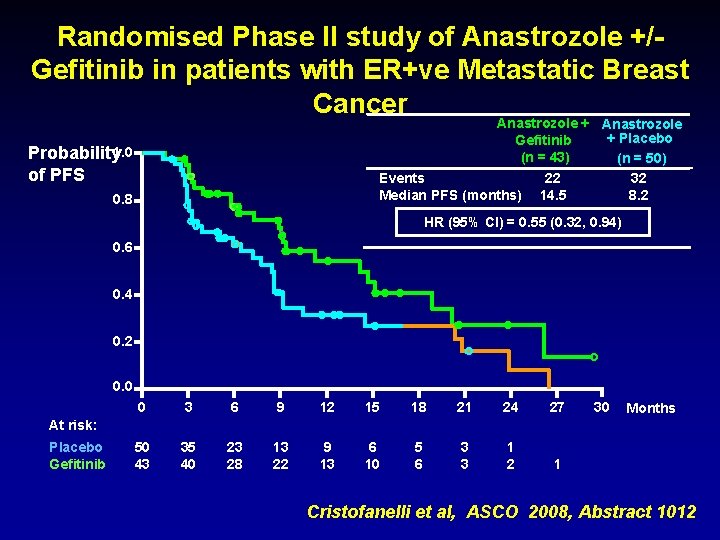
Randomised Phase II study of Anastrozole +/Gefitinib in patients with ER+ve Metastatic Breast Cancer Anastrozole + Placebo Gefitinib (n = 43) (n = 50) Events 22 32 Median PFS (months) 14. 5 8. 2 Probability 1. 0 of PFS 0. 8 HR (95% CI) = 0. 55 (0. 32, 0. 94) 0. 6 0. 4 0. 2 0. 0 0 3 6 9 12 15 18 21 24 27 50 43 35 40 23 28 13 22 9 13 6 10 5 6 3 3 1 2 1 30 Months At risk: Placebo Gefitinib Cristofanelli et al, ASCO 2008, Abstract 1012

Wat is “targeted therapie”? Target = Predictieve biomerker Goed omschreven molecule ~ tumorgroei/overlev -Molecule in normaal biologisch proces -Molecule in alle kankers -Molecule in sommige kankers (goed meetbaar) Molecule uitschakelen ~ Therapeutisch effect






Conclusions l Iniparib + GC no improved PFS or OS l Exploratory analyses of PFS and OS by prior therapy suggests: – Potential efficacy benefit among 2 nd/3 rd line patients. – Confirmatory study needed. l GCI safety profile confirmed; toxicity comparable to GC arm. l m. TNBC population is highly heterogeneous on intrinsic subtyping. l Biomarker analyses underway to evaluate patient populations that may benefit from iniparib. O’Shaughnessy J et al. Proc ASCO 2011; Abstract 1007.

e Som TN ted a HR l r u g o f pre ed RP u A y P l t e e r ra gula and us y l e r n n ir Ro EGF IT is dow NA repa K n D -ness i t c efe BRCA d e av BC h

Allred score 0 X Allred score 4 1 + 3 0 + 0 Allred score 7 5 + 2 ER IHC Allred score 8 5 + 3

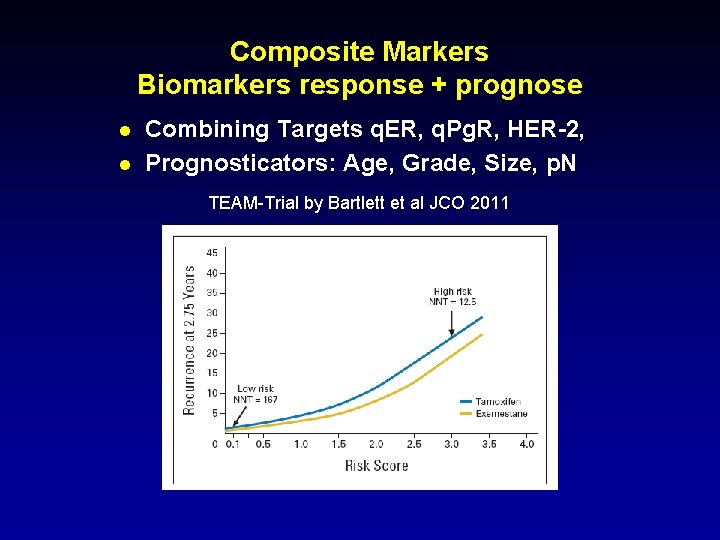
Composite Markers Biomarkers response + prognose l l Combining Targets q. ER, q. Pg. R, HER-2, Prognosticators: Age, Grade, Size, p. N TEAM-Trial by Bartlett et al JCO 2011

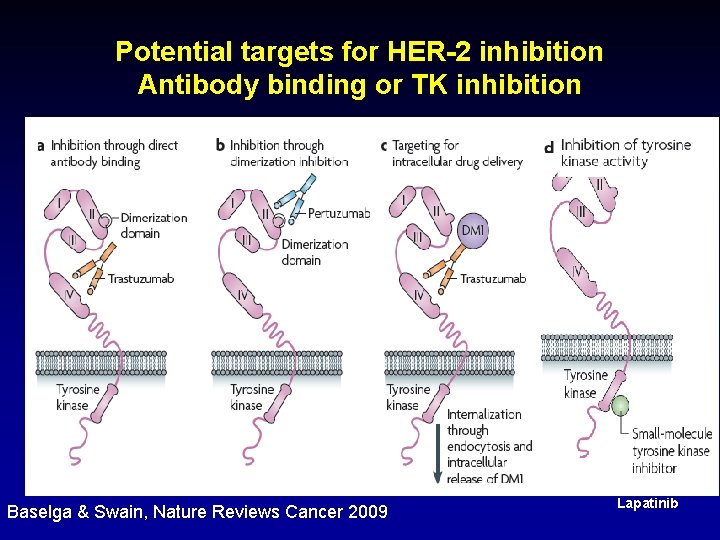
Potential targets for HER-2 inhibition Antibody binding or TK inhibition Baselga & Swain, Nature Reviews Cancer 2009 Lapatinib

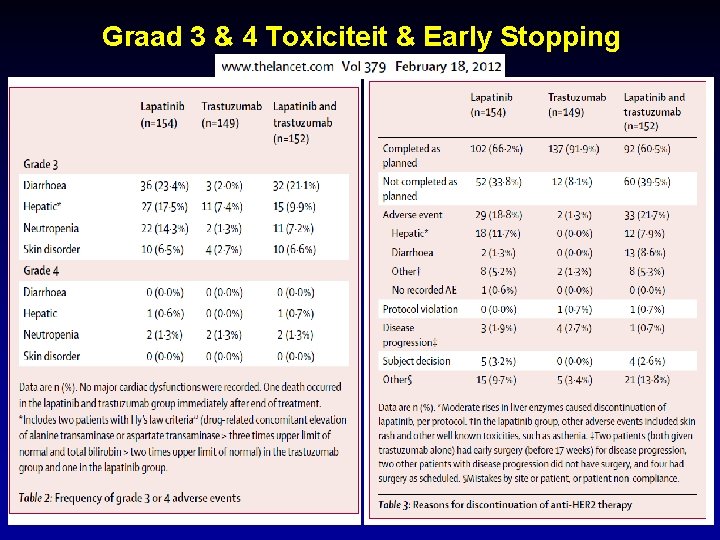
Graad 3 & 4 Toxiciteit & Early Stopping


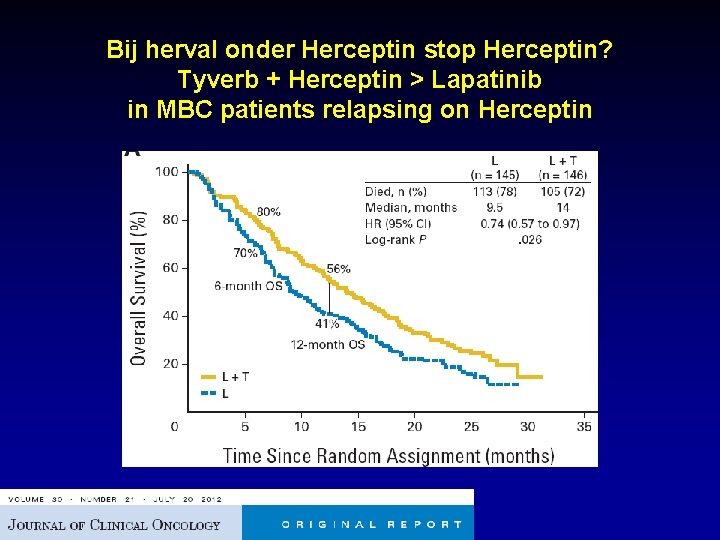
Bij herval onder Herceptin stop Herceptin? Tyverb + Herceptin > Lapatinib in MBC patients relapsing on Herceptin

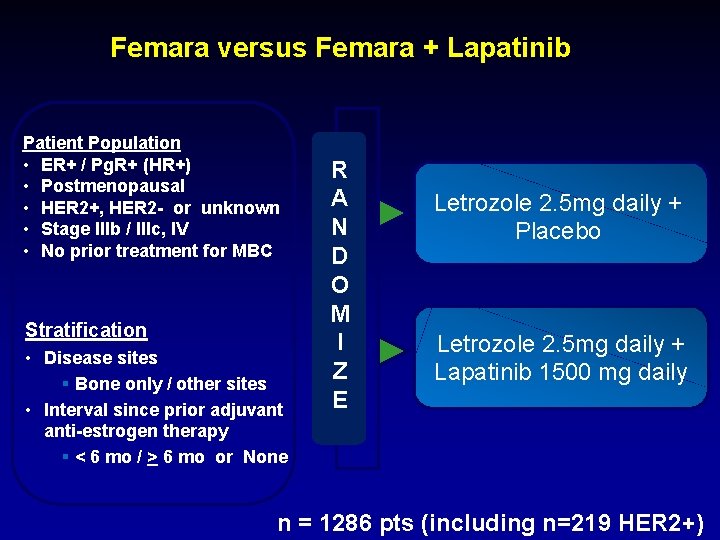
Femara versus Femara + Lapatinib Patient Population • ER+ / Pg. R+ (HR+) • Postmenopausal • HER 2+, HER 2 - or unknown • Stage IIIb / IIIc, IV • No prior treatment for MBC Stratification • Disease sites § Bone only / other sites • Interval since prior adjuvant anti-estrogen therapy § < 6 mo / > 6 mo or None R A N D O M I Z E Letrozole 2. 5 mg daily + Placebo Letrozole 2. 5 mg daily + Lapatinib 1500 mg daily n = 1286 pts (including n=219 HER 2+)

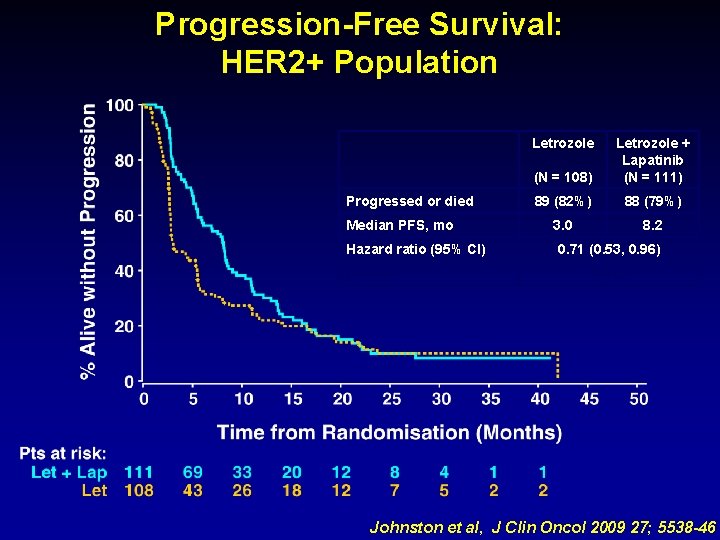
Progression-Free Survival: HER 2+ Population (N = 108) Letrozole + Lapatinib (N = 111) 89 (82%) 88 (79%) 3. 0 8. 2 Letrozole Progressed or died Median PFS, mo Hazard ratio (95% CI) p-value 0. 71 (0. 53, 0. 96) 0. 019 Johnston et al, J Clin Oncol 2009 27; 5538 -46


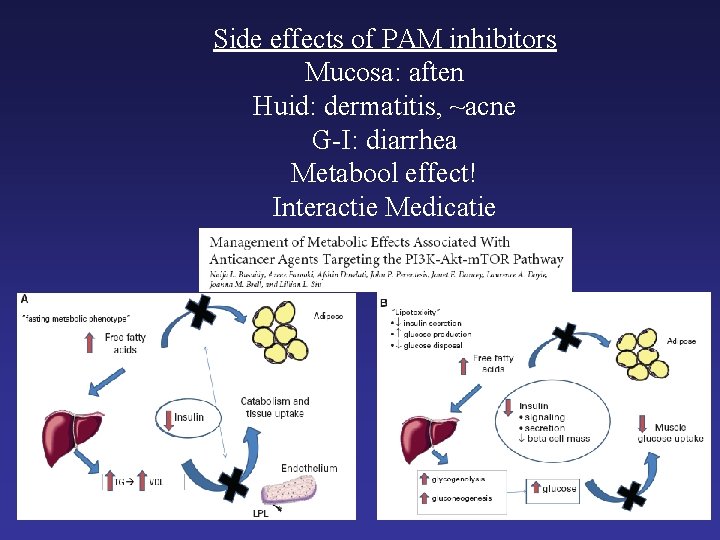
Side effects of PAM inhibitors Mucosa: aften Huid: dermatitis, ~acne G-I: diarrhea Metabool effect! Interactie Medicatie

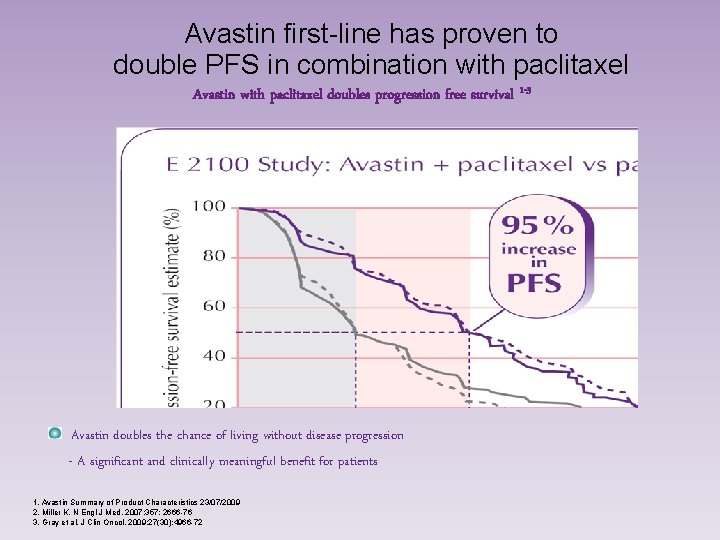
Avastin first-line has proven to double PFS in combination with paclitaxel Avastin with paclitaxel doubles progression free survival 1 -3 Avastin doubles the chance of living without disease progression - A significant and clinically meaningful benefit for patients 1. Avastin Summary of Product Characteristics 23/07/2009 2. Miller K. N Engl J Med. 2007; 357: 2666 -76 3. Gray et al. J Clin Oncol. 2009; 27(30): 4966 -72

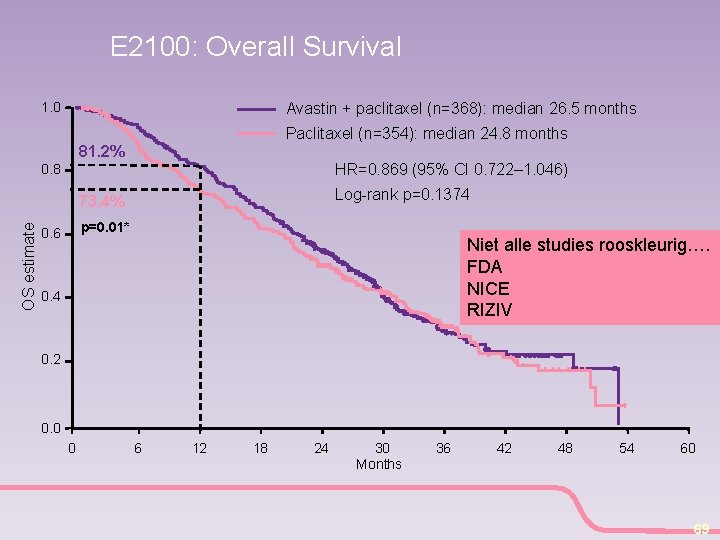
E 2100: Overall Survival 1. 0 Avastin + paclitaxel (n=368): median 26. 5 months Paclitaxel (n=354): median 24. 8 months 81. 2% HR=0. 869 (95% CI 0. 722– 1. 046) 0. 8 Log-rank p=0. 1374 OS estimate 73. 4% p=0. 01* 0. 6 Niet alle studies rooskleurig…. FDA NICE RIZIV 0. 4 0. 2 0. 0 0 6 12 18 24 30 Months 36 42 48 54 60 69
 Systematic desensitization therapy
Systematic desensitization therapy Rankl antibody
Rankl antibody Angiogênese
Angiogênese Comparative and superlative diferencias
Comparative and superlative diferencias His my
His my Mine his hers
Mine his hers Sources of recruitment in hrm
Sources of recruitment in hrm Targeted early numeracy (ten) intervention program
Targeted early numeracy (ten) intervention program Ncach
Ncach Consist of your most important targeted or segmented groups
Consist of your most important targeted or segmented groups Targeted disabilities
Targeted disabilities Targeted delivery
Targeted delivery Targeted solutions tool
Targeted solutions tool Mcos cover south gsa
Mcos cover south gsa Marketing involve engaging directly with carefully targeted
Marketing involve engaging directly with carefully targeted Neogov
Neogov Nerviano medical sciences
Nerviano medical sciences Targeted local hire program
Targeted local hire program Marketing involve engaging directly with carefully targeted
Marketing involve engaging directly with carefully targeted Mshda targeted areas
Mshda targeted areas Targeted youth support islington
Targeted youth support islington Public candy companies
Public candy companies Targeted local hire
Targeted local hire Psychodynamic and humanistic therapies have in common
Psychodynamic and humanistic therapies have in common What is biomedical therapy
What is biomedical therapy Bodywork and movement therapies
Bodywork and movement therapies Flooding therapy
Flooding therapy Advanced therapies apprenticeship community
Advanced therapies apprenticeship community Pharmacological and parenteral therapies
Pharmacological and parenteral therapies Biomedical therapy definition
Biomedical therapy definition Stiriti ayur therapies
Stiriti ayur therapies Insight therapies involve verbal interactions
Insight therapies involve verbal interactions Chapter 32 complementary and alternative therapies
Chapter 32 complementary and alternative therapies Trafford iapt
Trafford iapt Trafford psychological therapies
Trafford psychological therapies Astellas gene therapies
Astellas gene therapies Talking therapies westminster
Talking therapies westminster Trafford psychological therapies
Trafford psychological therapies Psychoanalytic therapy is to as humanistic therapy is to
Psychoanalytic therapy is to as humanistic therapy is to Module 73: the biomedical therapies
Module 73: the biomedical therapies Eight limbs of yoga ppt
Eight limbs of yoga ppt Bodywork and movement therapies
Bodywork and movement therapies Trafford psychological therapies
Trafford psychological therapies Biomedical therapy techniques
Biomedical therapy techniques Advantages of group therapy
Advantages of group therapy