Target Product Claims in CancerAssociated Fatigue Target Product











- Slides: 13

Target Product Claims in Cancer-Associated Fatigue

Target Product Claims Target product claims are a portion of the target product profile (TPP) approach to drug development n A TPP describes the profile of the drug similar to a package insert. n A Clinical Development Plan describes the program of studies to achieve/support the TPP n

TPP vs TPC n The TPP and TPC approach differs from hypothesis-based research. The end result is described and the program is built around it. n The TPP/TPC is a “living” document and can be altered based on new data n

Claim List for Fatigue Therapy Claim Clinical trial issues Comments Stimulin™ is indicated for the treatment of the symptoms of fatigue associated with cancer n Can/must be assessed n The drug is not addressing independent of therapy n Baseline assessment needed? the root cause of fatigue – just influencing the reported symptoms. Influenced by concomitant treatments Can be assessed after Subgroups? treatment but etiology is a question. Treatment of fatigue Clinical trials require baseline This claim would require data before cancer therapy. Would need to know the relative incidence of treatable fatigue after a given cancer therapy to determine sample size. near total absence of fatigue in subjects at study end. This implies a treatment of the More difficult claim because entire fatigue spectrum. Higher hurdle for clinical trials. the source of the fatigue is obscured by treatment Stimulin™ is indicated for the treatment of the symptoms of fatigue associated with cancer therapy (chemotherapy or radiotherapy, etc. ) Stimulin is indicated for the prevention of fatigue symptoms associated with cancer therapy (chemotherapy or radiotherapy) Stimulin is indicated for the treatment of the fatigue associated with cancer. symptoms vs treatment of fatigue

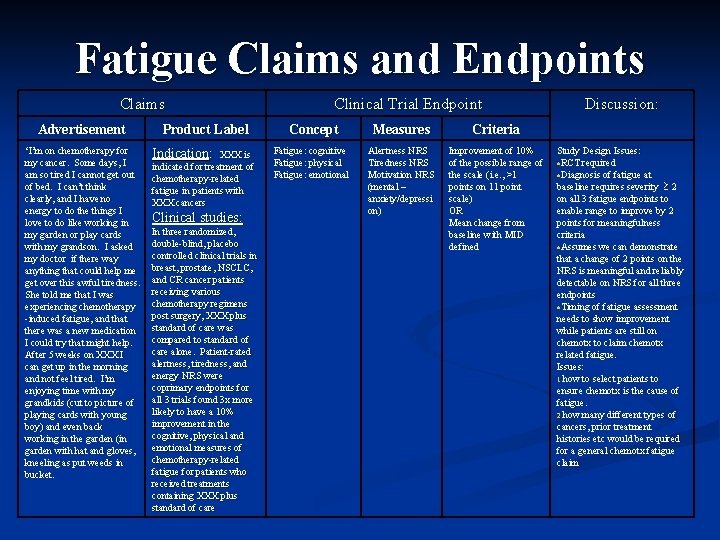
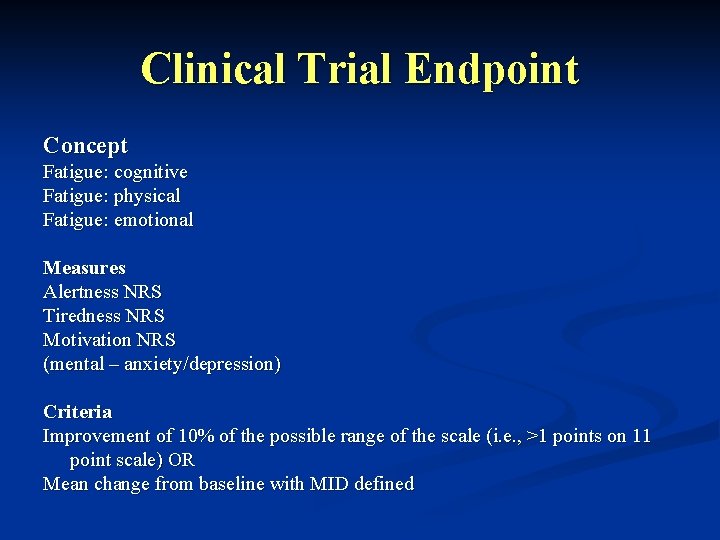
Fatigue Claims and Endpoints Claims Advertisement ‘I’m on chemotherapy for my cancer. Some days, I am so tired I cannot get out of bed. I can’t think clearly, and I have no energy to do the things I love to do like working in my garden or play cards with my grandson. I asked my doctor if there way anything that could help me get over this awful tiredness. She told me that I was experiencing chemotherapy -induced fatigue, and that there was a new medication I could try that might help. After 5 weeks on XXX I can get up in the morning and not feel tired. I’m enjoying time with my grandkids (cut to picture of playing cards with young boy) and even back working in the garden (in garden with hat and gloves, kneeling as put weeds in bucket. Product Label Indication: XXX is indicated for treatment of chemotherapy-related fatigue in patients with XXX cancers Clinical studies: In three randomized, double-blind, placebo controlled clinical trials in breast, prostate, NSCLC, and CR cancer patients receiving various chemotherapy regimens post surgery, XXX plus standard of care was compared to standard of care alone. Patient-rated alertness, tiredness, and energy NRS were coprimary endpoints for all 3 trials found 3 x more likely to have a 10% improvement in the cognitive, physical and emotional measures of chemotherapy-related fatigue for patients who received treatments containing XXX plus standard of care Clinical Trial Endpoint Concept Measures Criteria Fatigue: cognitive Fatigue: physical Fatigue: emotional Alertness NRS Tiredness NRS Motivation NRS (mental – anxiety/depressi on) Improvement of 10% of the possible range of the scale (i. e. , >1 points on 11 point scale) OR Mean change from baseline with MID defined Discussion: Study Design Issues: RCT required Diagnosis of fatigue at baseline requires severity 2 on all 3 fatigue endpoints to enable range to improve by 2 points for meaningfulness criteria Assumes we can demonstrate that a change of 2 points on the NRS is meaningful and reliably detectable on NRS for all three endpoints Timing of fatigue assessment needs to show improvement while patients are still on chemotx to claim chemotx related fatigue. Issues: 1. how to select patients to ensure chemotx is the cause of fatigue. 2. how many different types of cancers, prior treatment histories etc would be required for a general chemotx fatigue claim

Questions for Discussion n How can we deal with the confounding sources of fatigue? n Depression/ mood disorder n Cancer treatment n Tumor n Surgery n Concomitant medication n Radiation

Questions for Discussion n Are the symptoms of fatigue unidimensional or multidimensional? n If yes will there be subclaims for individual components? n If multidimensional – what would the impact be on claim language and fatigue measurement? n Claim for improvement in function n Claim for subjective improvement in cognitive aspects of fatigue n Claim for subjective improvement in mental aspects of fatigue (other than cognition)

Questions for Discussion What degree of improvement justifies the claim? MID? how to assess? n Is it feasible to prevent fatigue? n n n How can it be studied? How do we limit the claim to cancer and not allow “claim creep” where chronic fatigue syndrome, liver disease etc may be treated without evidence?


Advertisement ‘I’m on chemotherapy for my cancer. Some days, I am so tired I cannot get out of bed. I can’t think clearly, and I have no energy to do the things I love to do like working in my garden or play cards with my grandson. I asked my doctor if there way anything that could help me get over this awful tiredness. She told me that I was experiencing chemotherapyinduced fatigue, and that there was a new medication I could try that might help. After 5 weeks on XXX I can get up in the morning and not feel tired. I’m enjoying time with my grandkids (cut to picture of playing cards with young boy) and even back working in the garden (in garden with hat and gloves, kneeling as put weeds in bucket.

Product Label Indication: XXX is indicated for treatment of chemotherapy-related fatigue in patients with XXX cancers Clinical studies: In three randomized, double-blind, placebo controlled clinical trials in breast, prostate, NSCLC, and CR cancer patients receiving various chemotherapy regimens post surgery, XXX plus standard of care was compared to standard of care alone. Patient-rated alertness, tiredness, and energy NRS were coprimary endpoints for all 3 trials found 3 x more likely to have a 10% improvement in the cognitive, physical and emotional measures of chemotherapy-related fatigue for patients who received treatments containing XXX plus standard of care

Clinical Trial Endpoint Concept Fatigue: cognitive Fatigue: physical Fatigue: emotional Measures Alertness NRS Tiredness NRS Motivation NRS (mental – anxiety/depression) Criteria Improvement of 10% of the possible range of the scale (i. e. , >1 points on 11 point scale) OR Mean change from baseline with MID defined

Study Design Issues RCT required Diagnosis of fatigue at baseline requires severity 2 on all 3 fatigue endpoints to enable range to improve by 2 points for meaningfulness criteria Assumes we can demonstrate that a change of 2 points on the NRS is meaningful and reliably detectable on NRS for all three endpoints Timing of fatigue assessment needs to show improvement while patients are still on chemotx to claim chemotx related fatigue. Issues: 1. how to select patients to ensure chemotx is the cause of fatigue. 2. how many different types of cancers, prior treatment histories etc would be required for a general chemotx fatigue claim
 Target product profile drug example
Target product profile drug example Effective persuasive claim letters
Effective persuasive claim letters Primary target market and secondary target market
Primary target market and secondary target market Alarm fatigue definition
Alarm fatigue definition Corrections fatigue
Corrections fatigue What is fatigue failure
What is fatigue failure Employee fatigue training
Employee fatigue training Powerpoint template
Powerpoint template Fibromyalgia vs chronic fatigue
Fibromyalgia vs chronic fatigue Slides fatigue test
Slides fatigue test System shock fatigue
System shock fatigue Objective of stress management
Objective of stress management Gerber equation fatigue
Gerber equation fatigue Desiderata by max
Desiderata by max

























