Take out your notes and answer the warm
















- Slides: 27

Take out your notes and answer the warm up questions… * What particle in the atom do you think is allowed to move around? * What do you think you find inside neon lights?

How does light behave? Wave-Particle Duality

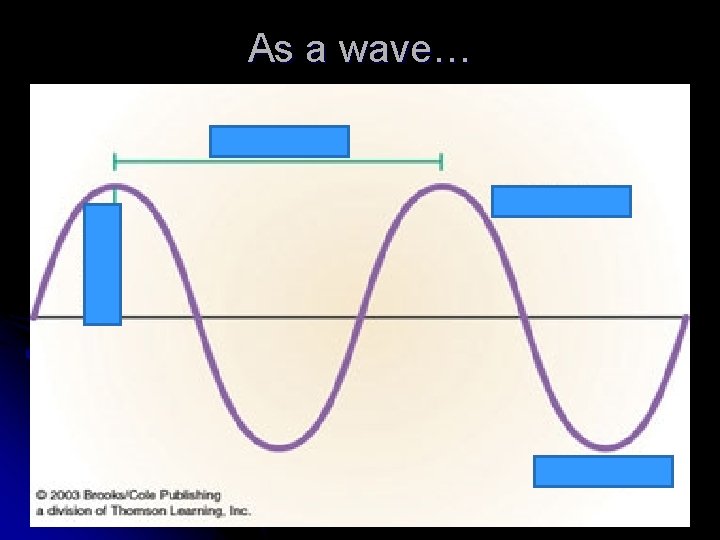
As a wave…

l Wavelength (λ): shortest distance between 2 equivalent points on a wave l Frequency ( ): number of waves that pass a given point per second l All electromagnetic waves travel at the same speed (in a vacuum)

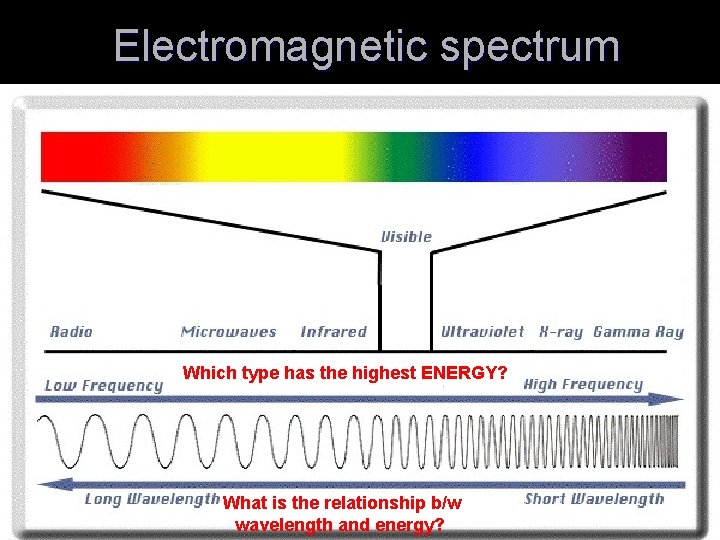
Electromagnetic spectrum Which type has the highest ENERGY? What is the relationship b/w wavelength and energy?


Atomic Emission Spectra An element’s spectral thumbprint l l l The set of frequencies given off by the atoms of a given element When electrons are excited, (from an outside source) they emit certain frequencies Each element’s spectra is unique

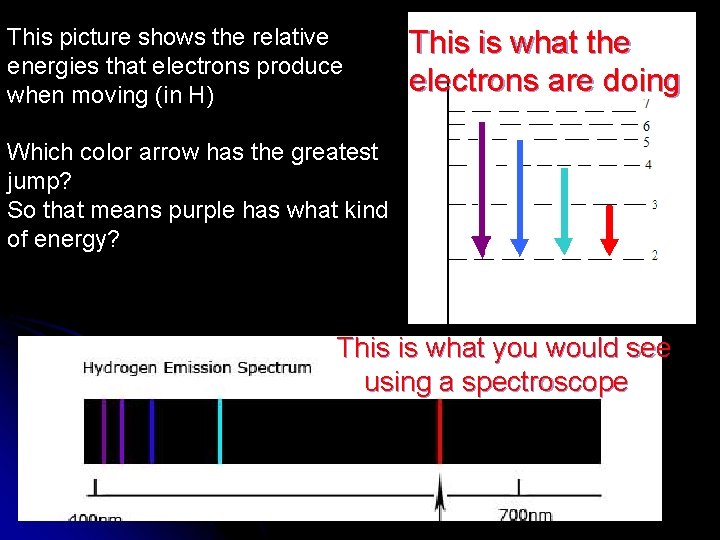
This picture shows the relative energies that electrons produce when moving (in H) This is what the electrons are doing Which color arrow has the greatest jump? So that means purple has what kind of energy? This is what you would see using a spectroscope

As a particle… l Photoelectric effect – when electrons are emitted from a metal’s surface when a certain frequency shines on it l Light is a form of energy that can be released by an atom, and is made up of small particles of energy called photons.

Photoelectric effect Photons – small particles of energy Electrons ejected from the surface Sodium metal If the light has a higher frequency, it will also have a higher _______, and this effects the number of electrons emitted.

Warm up… l As wavelength increases, frequency _____ and energy ____ l Which color of visible light has the lowest energy? l Draw the Bohr model for Sulfur

Heisenberg may have slept here… l Heisenberg uncertainty principle – States that it is impossible to know precisely both the velocity and position of an electron at the same time e-

Now it’s a wave… l l Schrödinger came up with a very difficult equation which treated electrons as waves The wave function he calculated is related to the probability of finding electrons within a certain volume of space around the nucleus

Atomic Orbitals l Principal energy levels (n) l Each energy levels contains particular sublevels: s, p, d or f l Each sublevel has a specific number of orbitals; each orbital can hold only ____ electrons

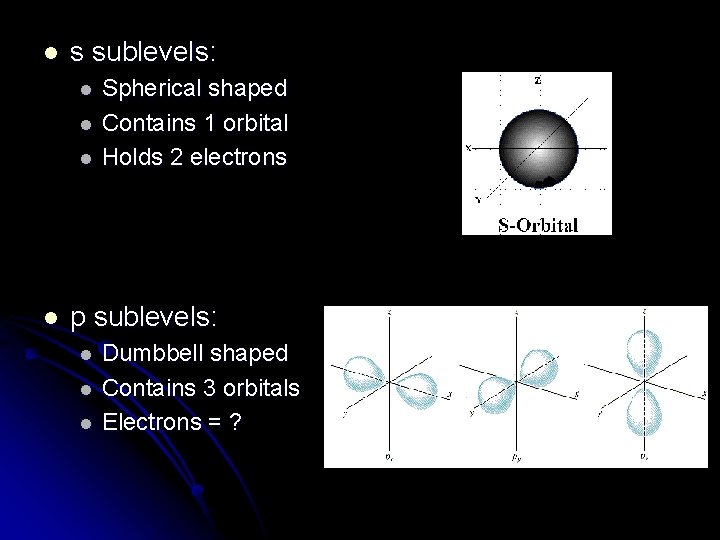
l s sublevels: l l Spherical shaped Contains 1 orbital Holds 2 electrons p sublevels: l l l Dumbbell shaped Contains 3 orbitals Electrons = ?

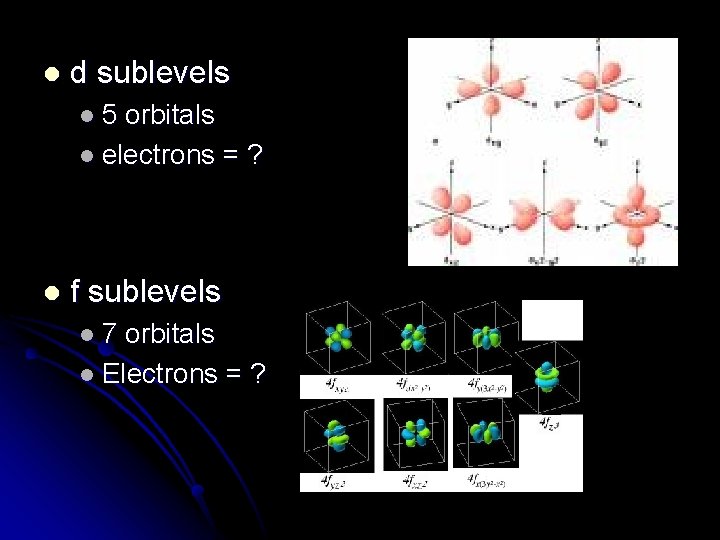
l d sublevels l 5 orbitals l electrons = ? l f sublevels l 7 orbitals l Electrons = ?

HOTEL PINO There are 7 levels (principle energy levels) Types of rooms (sublevels): special premium deluxe fabulous

# of beds (orbitals) special – 1 bed premium – 3 beds deluxe – 5 beds fabulous – 7 beds 2 people maximum per bed

Bosses and their rules Bohr (AKA Aufbau) “You must fill a room before moving on to the next, and you must fill from the bottom of the hotel to the top” l Hund – “No doubling up until each bed in a room is occupied” l Pauli – “Only 2 people per bed, sleeping in opposite directions” l

Hund’s rule AKA the bus rule l If you are going to sit on a bus with strangers, will you more than likely sit next to a stranger, or take an empty seat? l Hund’s rule is the same: one electron must occupy an orbital (seat) in a sublevel (room) until they absolutely have to start doubling up

Take out the notes from yesterday… Warm up: Write 0. 0000673 in scientific notation How many protons, neutrons and electrons are in Aluminum-29? Is salt dissolving in water a physical or chemical change?

Aufbau diagram • Always start at the top of the diagram, and then work your way down each arrow S sublevels have a max of 2 electrons P sublevels have a max of 6 electrons How many for d and f? look back at notes

Electron configurations l A symbolic notation of how electrons are arranged in an atom Figure out your stopping point, and then use the Aufbau diagram

Orbital diagrams Similar to electron configurations, but shows a more specific arrangement of the electrons l Boxes or lines are used to represent the orbitals (beds) l Each set of orbitals is labeled with the energy level and sublevel l Arrows are used to represent electrons l

l How many lines (orbitals) will you have for an s-sublevel? P- sublevel? D? F? l 1, 3, 5, 7 l You must keep the 3 rules in mind when doing orbital diagrams l Write out the electron configurations for oxygen and silicon

Valence electrons l Electrons on the outermost orbitals l Write the electron (or noble gas) configurations for calcium and bromine l What is the highest number energy level? How many electrons are on it? l Careful with bromine!

Lewis dot structures l Show only the valence electrons l Follow Hund’s rule when drawing the dots, don’t start pairing up until you have to