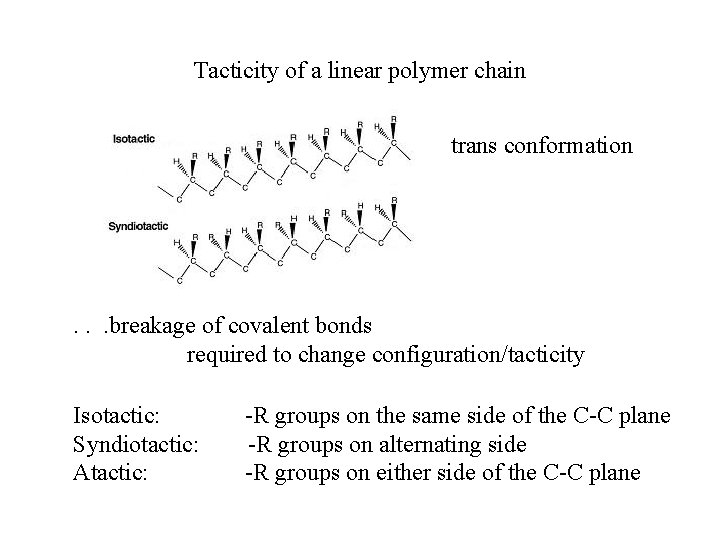
Tacticity of a linear polymer chain trans conformation


- Slides: 13

Tacticity of a linear polymer chain trans conformation . . . breakage of covalent bonds required to change configuration/tacticity Isotactic: Syndiotactic: Atactic: -R groups on the same side of the C-C plane -R groups on alternating side -R groups on either side of the C-C plane

States of polymers Gaseous Liquid Solid “melt”& solutions “glasses” semi-crystalline decrease temperature or thermal motion

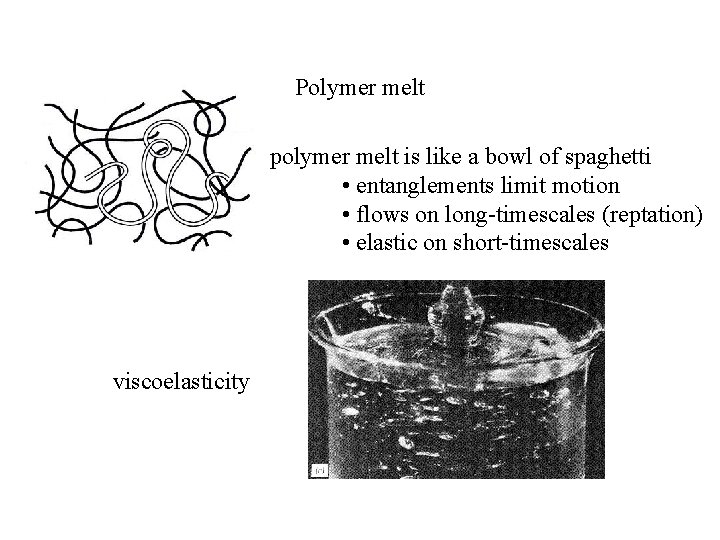
Polymer melt polymer melt is like a bowl of spaghetti • entanglements limit motion • flows on long-timescales (reptation) • elastic on short-timescales viscoelasticity

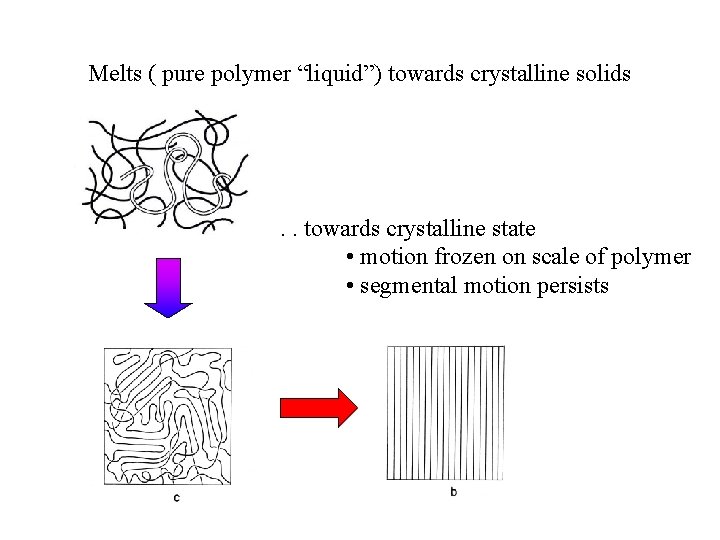
Melts ( pure polymer “liquid”) towards crystalline solids . . towards crystalline state • motion frozen on scale of polymer • segmental motion persists

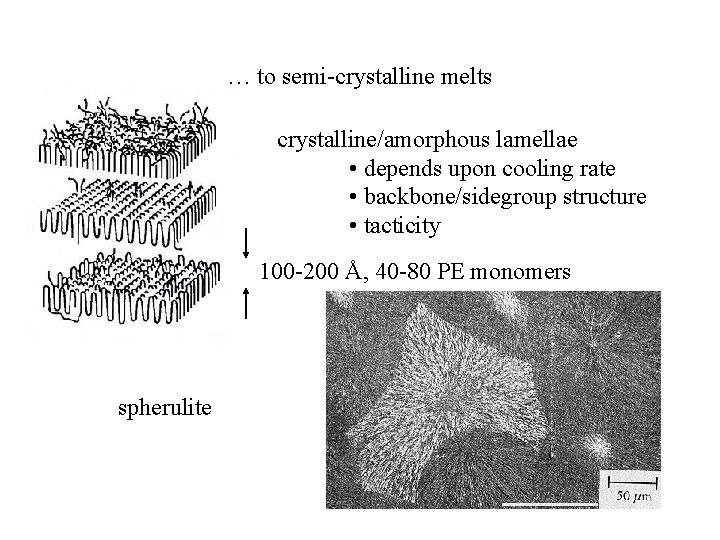
… to semi-crystalline melts crystalline/amorphous lamellae • depends upon cooling rate • backbone/sidegroup structure • tacticity 100 -200 Å, 40 -80 PE monomers spherulite

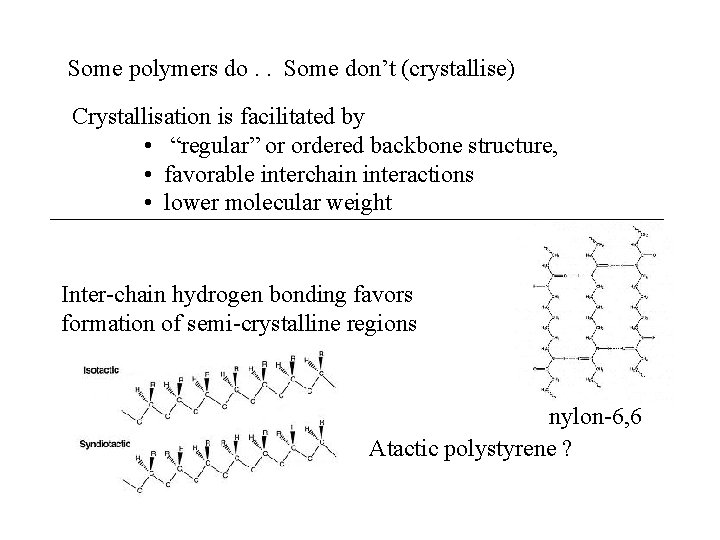
Some polymers do. . Some don’t (crystallise) Crystallisation is facilitated by • “regular” or ordered backbone structure, • favorable interchain interactions • lower molecular weight Inter-chain hydrogen bonding favors formation of semi-crystalline regions nylon-6, 6 Atactic polystyrene ?

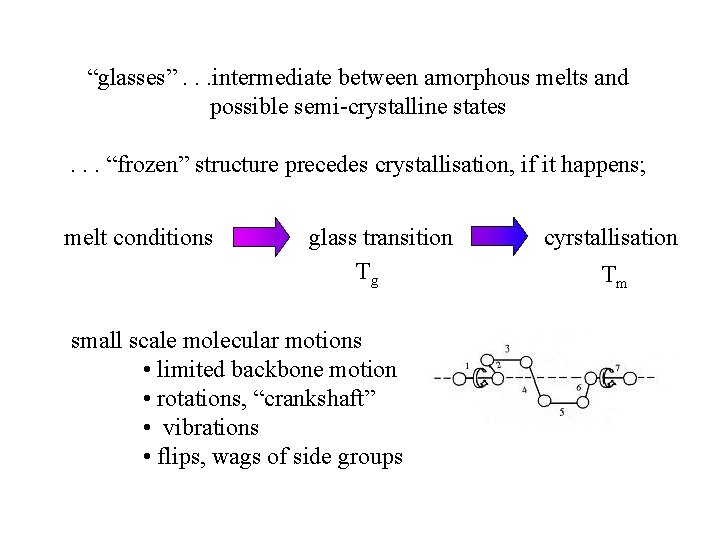
“glasses”. . . intermediate between amorphous melts and possible semi-crystalline states. . . “frozen” structure precedes crystallisation, if it happens; melt conditions glass transition Tg small scale molecular motions • limited backbone motion • rotations, “crankshaft” • vibrations • flips, wags of side groups cyrstallisation Tm

Q 4: Using complete sentences, and schematics if helpful, contrast the chain motion of a melt of atactic polypropylene under (a) slow temperature quench; (b) quick temperature quench Q 5: Complete Q 4 again, starting from a melt of nylon 6, 6

Q 6: Atactic-polyvinylchloride (PVC) is amorphous, whereas syndiotactic-PVC is partially cyrstalline. But atactic- polyvinylalcohol is partly crystalline. Why? Q 7: Do you predict the Tm of nylon-6, 6 to be higher or lower than that of polyethylene? Why?

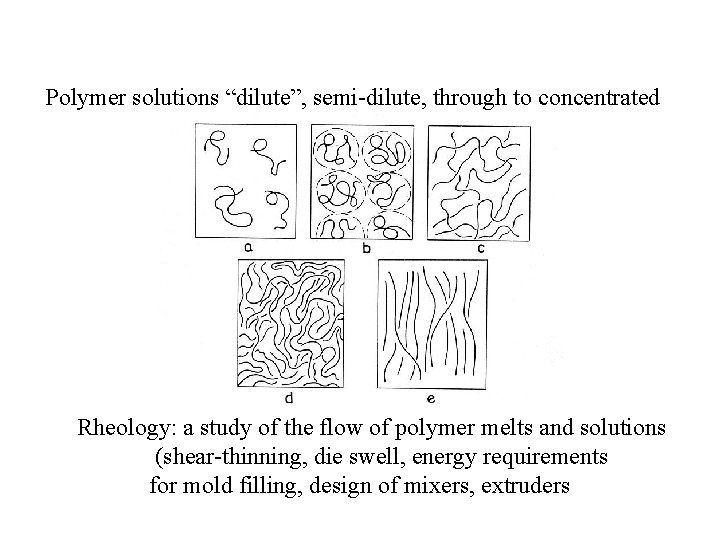
Polymer solutions “dilute”, semi-dilute, through to concentrated Rheology: a study of the flow of polymer melts and solutions (shear-thinning, die swell, energy requirements for mold filling, design of mixers, extruders

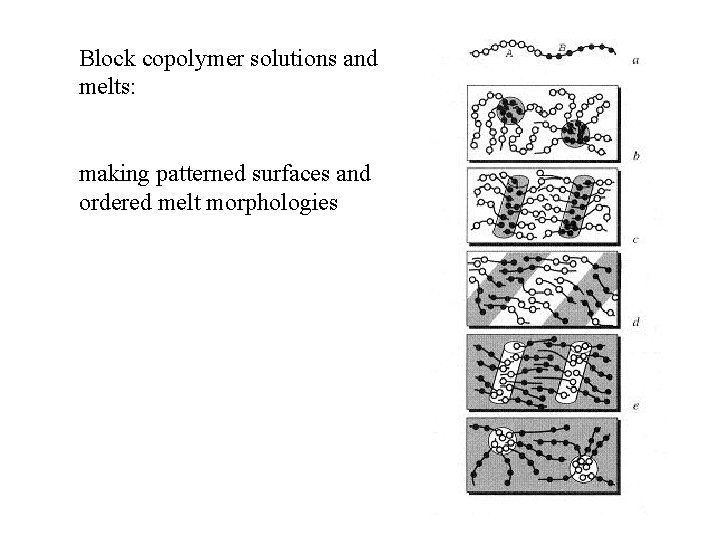
Block copolymer solutions and melts: making patterned surfaces and ordered melt morphologies

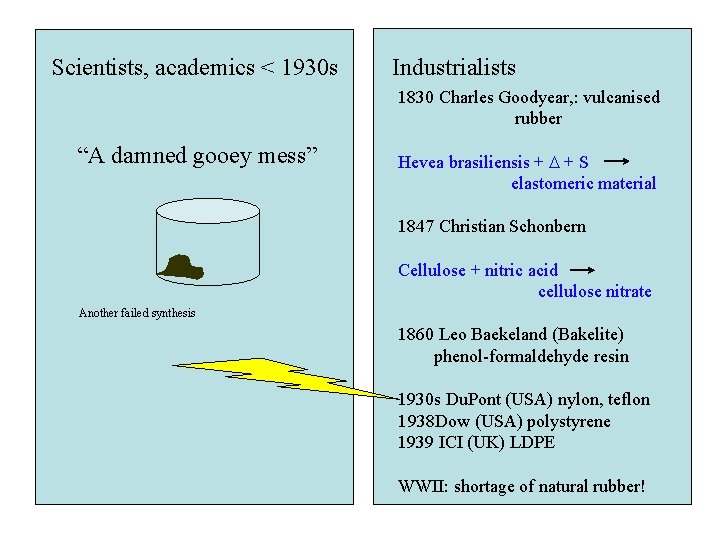
Scientists, academics < 1930 s Industrialists 1830 Charles Goodyear, : vulcanised rubber “A damned gooey mess” Hevea brasiliensis + D + S elastomeric material 1847 Christian Schonbern Cellulose + nitric acid cellulose nitrate Another failed synthesis 1860 Leo Baekeland (Bakelite) phenol-formaldehyde resin 1930 s Du. Pont (USA) nylon, teflon 1938 Dow (USA) polystyrene 1939 ICI (UK) LDPE WWII: shortage of natural rubber!

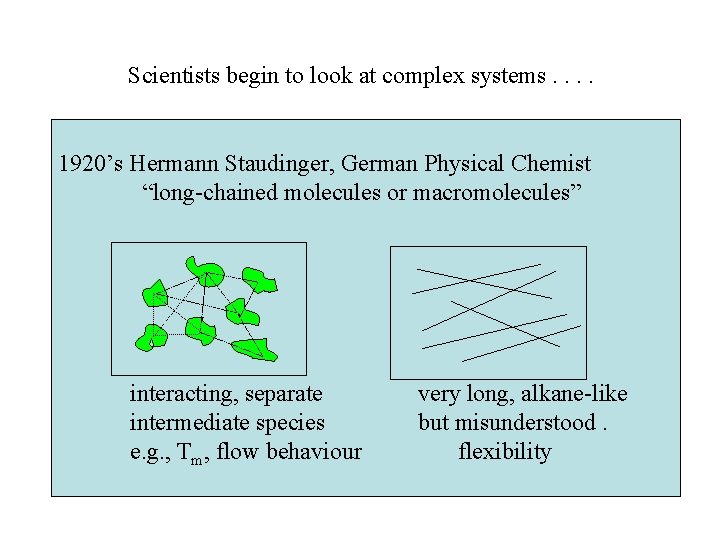
Scientists begin to look at complex systems. . 1920’s Hermann Staudinger, German Physical Chemist “long-chained molecules or macromolecules” interacting, separate intermediate species e. g. , Tm, flow behaviour very long, alkane-like but misunderstood. flexibility