Tackling the New Face of Prostate Cancer Amina




- Slides: 30

Tackling the New Face of Prostate Cancer Amina Zoubeidi, Ph. D. Professor, Dept. Urologic Sciences University of British Columbia Senior Scientist, Vancouver Prostate Centre @Amina. Zoubeidi PCF-BC January 2 nd, 2020

Disclosure I am a scientist not a clinician

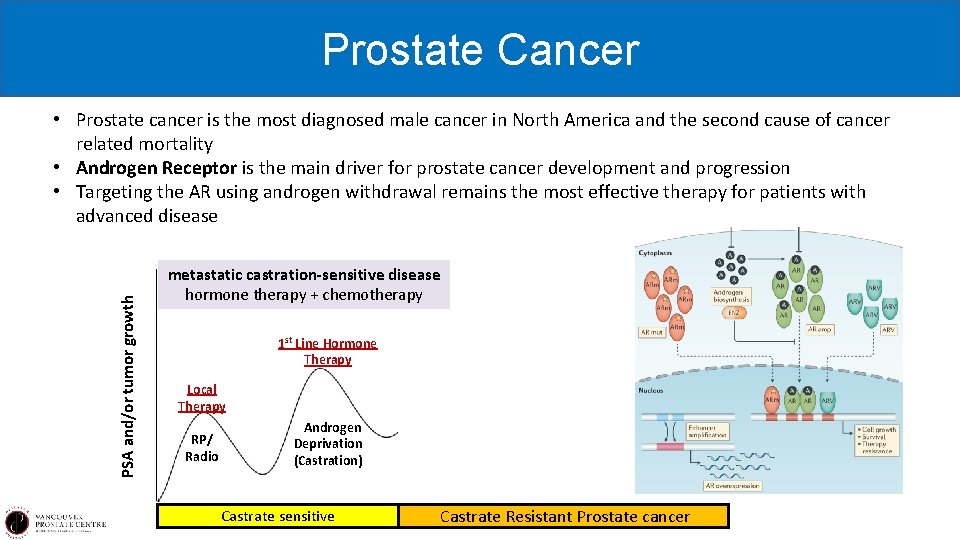
Prostate Cancer PSA and/or tumor growth • Prostate cancer is the most diagnosed male cancer in North America and the second cause of cancer related mortality • Androgen Receptor is the main driver for prostate cancer development and progression • Targeting the AR using androgen withdrawal remains the most effective therapy for patients with advanced disease metastatic castration-sensitive disease hormone therapy + chemotherapy 1 st Line Hormone Therapy Local Therapy RP/ Radio Androgen Deprivation (Castration) Castrate sensitive 2 nd Line Hormone Therapy 2012 2011 non metastatic CRPC Enzalutamide (PROSPER 2018) Apalutamide (SPARTAN 2018) Darolutamide (ARMIS 2019) Castrate Resistant Prostate cancer

However hormone therapy doesn’t work for every patients

Molecular Landscape of Advanced Prostate Cancer Genomic alterations are often heterogeneous across patients with metastatic castration-resistant prostate cancer. Different alterations can have distinct biological roles in driving disease progression, treatment response and resistance to therapies. By identifying and understanding each altered gene or pathway, we can guide unique therapeutic approaches for patients and improve clinical outcomes.

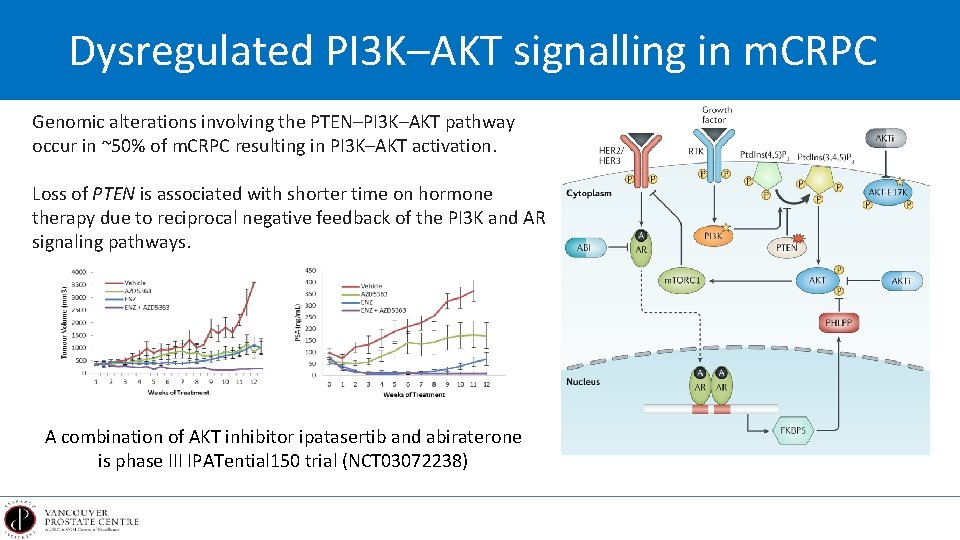
Dysregulated PI 3 K–AKT signalling in m. CRPC Genomic alterations involving the PTEN–PI 3 K–AKT pathway occur in ~50% of m. CRPC resulting in PI 3 K–AKT activation. Loss of PTEN is associated with shorter time on hormone therapy due to reciprocal negative feedback of the PI 3 K and AR signaling pathways. A combination of AKT inhibitor ipatasertib and abiraterone is phase III IPATential 150 trial (NCT 03072238)

DNA Repair Pathway DNA repair alterations (somatic or the germline) are observed in ~20% of m. CRPC Due to a high frequency of germline alterations: Germline testing is now recommended for patients with m. PCa

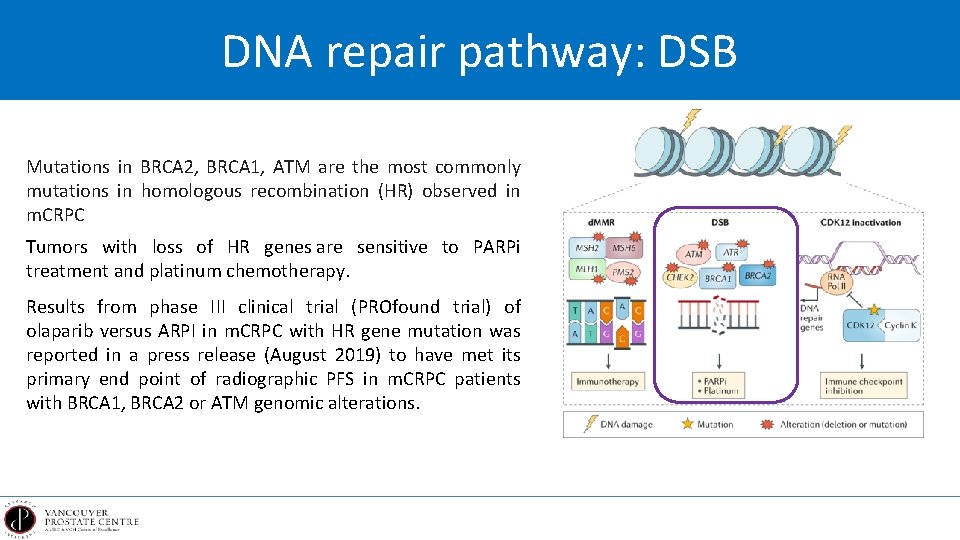
DNA repair pathway: DSB Mutations in BRCA 2, BRCA 1, ATM are the most commonly mutations in homologous recombination (HR) observed in m. CRPC Tumors with loss of HR genes are sensitive to PARPi treatment and platinum chemotherapy. Results from phase III clinical trial (PROfound trial) of olaparib versus ARPI in m. CRPC with HR gene mutation was reported in a press release (August 2019) to have met its primary end point of radiographic PFS in m. CRPC patients with BRCA 1, BRCA 2 or ATM genomic alterations.

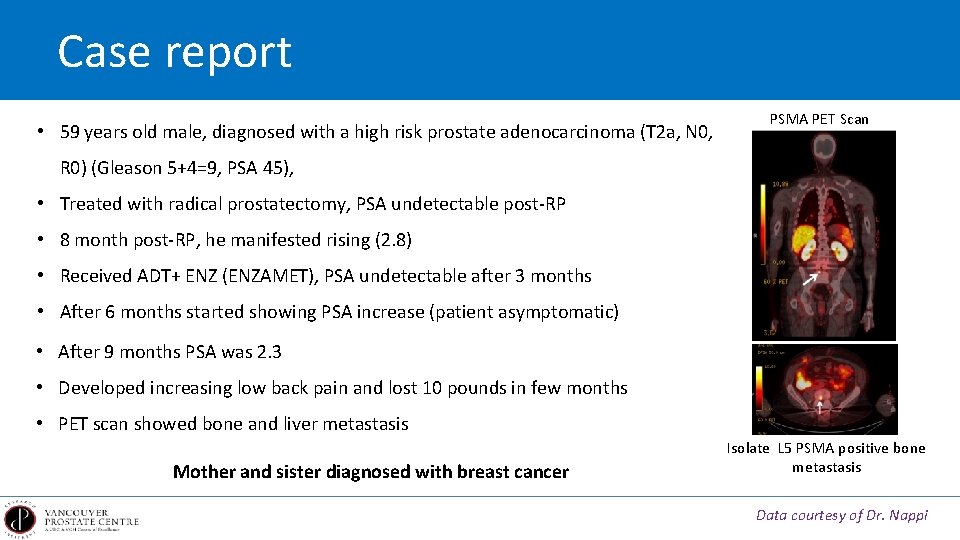
Case report • 59 years old male, diagnosed with a high risk prostate adenocarcinoma (T 2 a, N 0, PSMA PET Scan R 0) (Gleason 5+4=9, PSA 45), • Treated with radical prostatectomy, PSA undetectable post-RP • 8 month post-RP, he manifested rising (2. 8) • Received ADT+ ENZ (ENZAMET), PSA undetectable after 3 months • After 6 months started showing PSA increase (patient asymptomatic) • After 9 months PSA was 2. 3 • Developed increasing low back pain and lost 10 pounds in few months • PET scan showed bone and liver metastasis Mother and sister diagnosed with breast cancer Isolate L 5 PSMA positive bone metastasis Data courtesy of Dr. Nappi

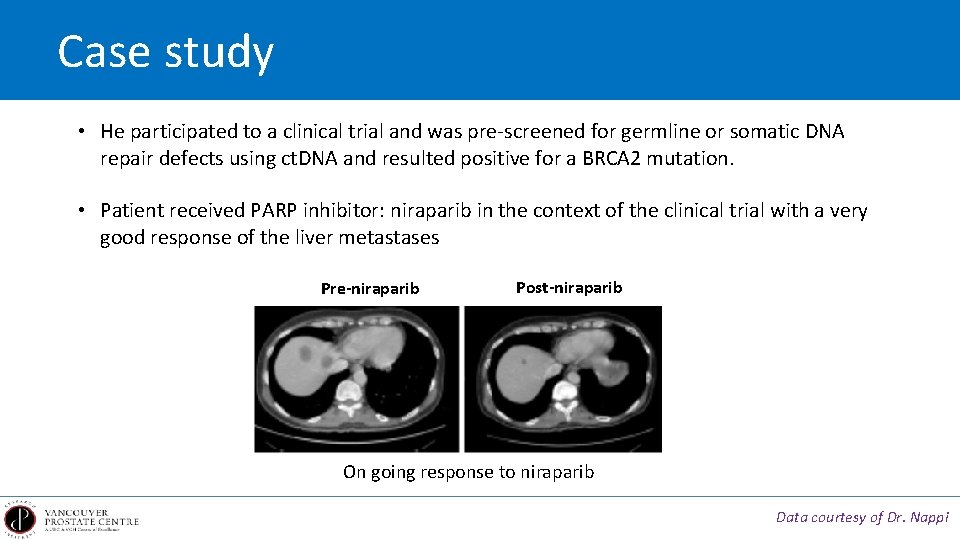
Case study • He participated to a clinical trial and was pre-screened for germline or somatic DNA repair defects using ct. DNA and resulted positive for a BRCA 2 mutation. • Patient received PARP inhibitor: niraparib in the context of the clinical trial with a very good response of the liver metastases Pre-niraparib Post-niraparib On going response to niraparib Data courtesy of Dr. Nappi

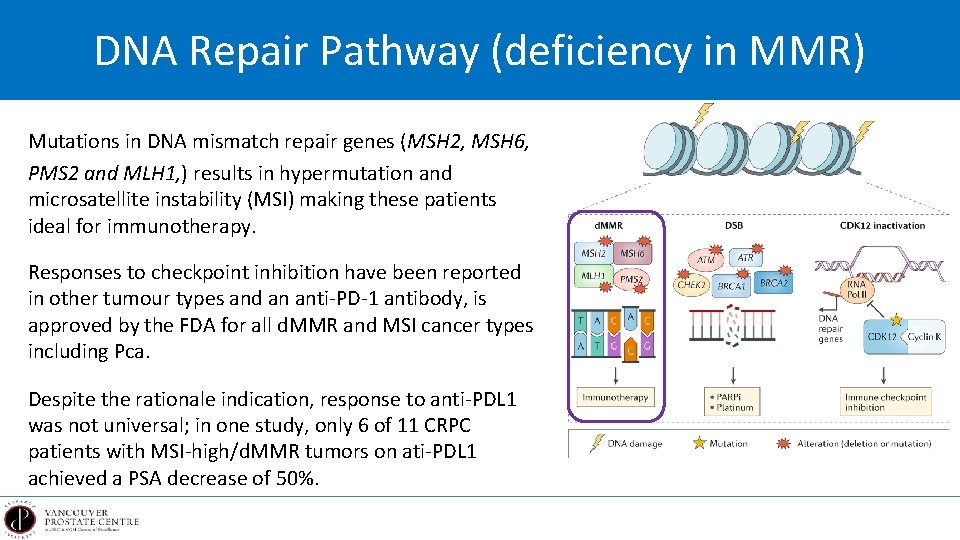
DNA Repair Pathway (deficiency in MMR) Mutations in DNA mismatch repair genes (MSH 2, MSH 6, PMS 2 and MLH 1, ) results in hypermutation and microsatellite instability (MSI) making these patients ideal for immunotherapy. Responses to checkpoint inhibition have been reported in other tumour types and an anti-PD-1 antibody, is approved by the FDA for all d. MMR and MSI cancer types including Pca. Despite the rationale indication, response to anti-PDL 1 was not universal; in one study, only 6 of 11 CRPC patients with MSI-high/d. MMR tumors on ati-PDL 1 achieved a PSA decrease of 50%.

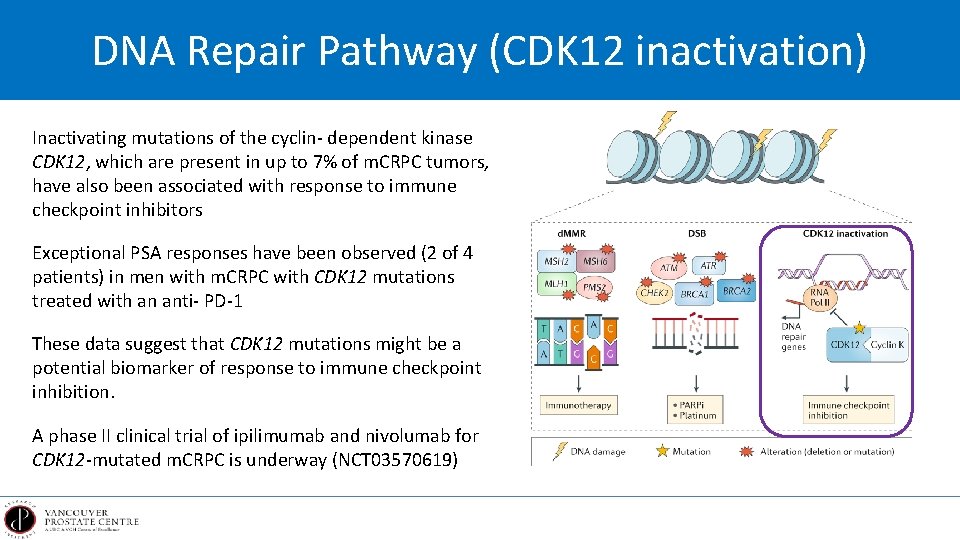
DNA Repair Pathway (CDK 12 inactivation) Inactivating mutations of the cyclin- dependent kinase CDK 12, which are present in up to 7% of m. CRPC tumors, have also been associated with response to immune checkpoint inhibitors Exceptional PSA responses have been observed (2 of 4 patients) in men with m. CRPC with CDK 12 mutations treated with an anti- PD-1 These data suggest that CDK 12 mutations might be a potential biomarker of response to immune checkpoint inhibition. A phase II clinical trial of ipilimumab and nivolumab for CDK 12 -mutated m. CRPC is underway (NCT 03570619)

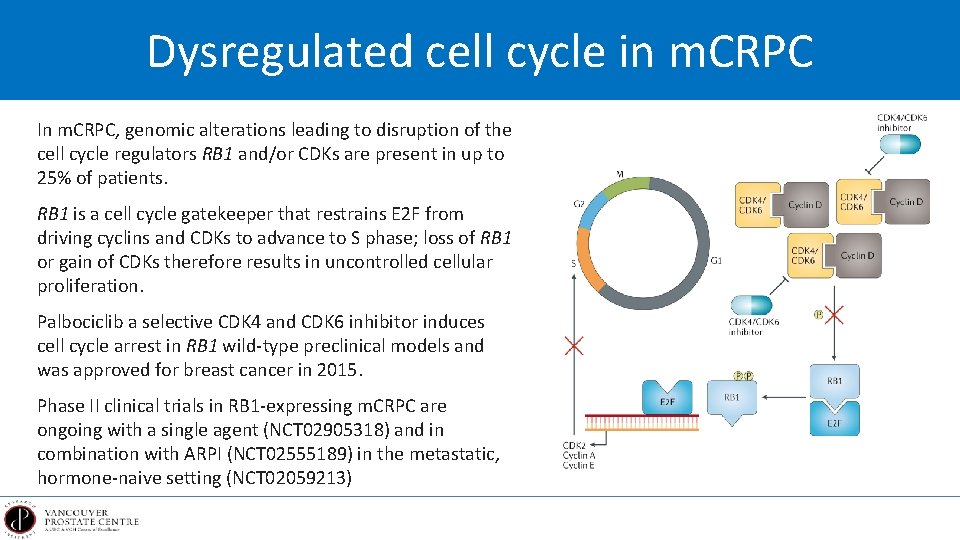
Dysregulated cell cycle in m. CRPC In m. CRPC, genomic alterations leading to disruption of the cell cycle regulators RB 1 and/or CDKs are present in up to 25% of patients. RB 1 is a cell cycle gatekeeper that restrains E 2 F from driving cyclins and CDKs to advance to S phase; loss of RB 1 or gain of CDKs therefore results in uncontrolled cellular proliferation. Palbociclib a selective CDK 4 and CDK 6 inhibitor induces cell cycle arrest in RB 1 wild-type preclinical models and was approved for breast cancer in 2015. Phase II clinical trials in RB 1 -expressing m. CRPC are ongoing with a single agent (NCT 02905318) and in combination with ARPI (NCT 02555189) in the metastatic, hormone-naive setting (NCT 02059213)

Resistance to nd 2 line hormone therapy

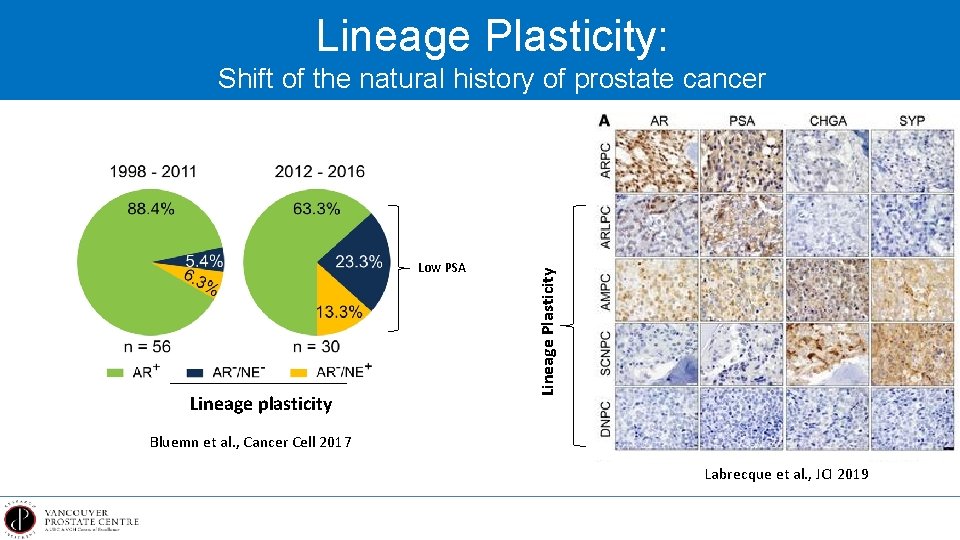
Lineage Plasticity: Low PSA Lineage plasticity Lineage Plasticity Shift of the natural history of prostate cancer Bluemn et al. , Cancer Cell 2017 Labrecque et al. , JCI 2019

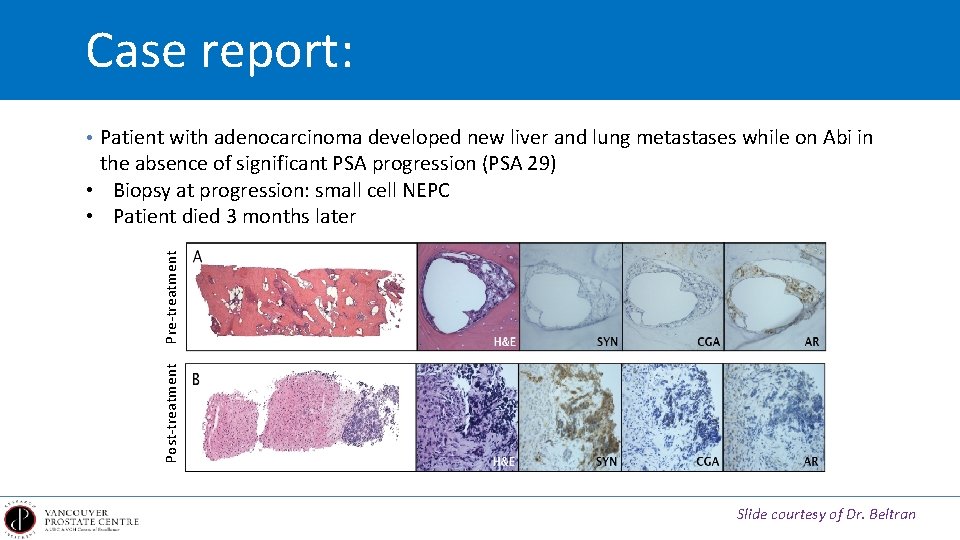
Case report: • Patient with adenocarcinoma developed new liver and lung metastases while on Abi in Post-treatment Pre-treatment the absence of significant PSA progression (PSA 29) • Biopsy at progression: small cell NEPC • Patient died 3 months later Slide courtesy of Dr. Beltran

What we call it? • Neuroendocrine Prostate Cancer (NEPC) Issue: Less aggressive compared to small cell • Small Cell Prostate Cancer Issue: Similar to the small cell • Aggressive Variant of Prostate Cancer Issue: Clinically relevant term but heterogeneous group • Androgen Indifferent Prostate Issue: AR continues to be expressed and some tumors my still respond to ARPI • Anaplastic Prostate Cancer Issue: Reflect pleomorphic cytology Adapted from Eric Small, PCF 2019

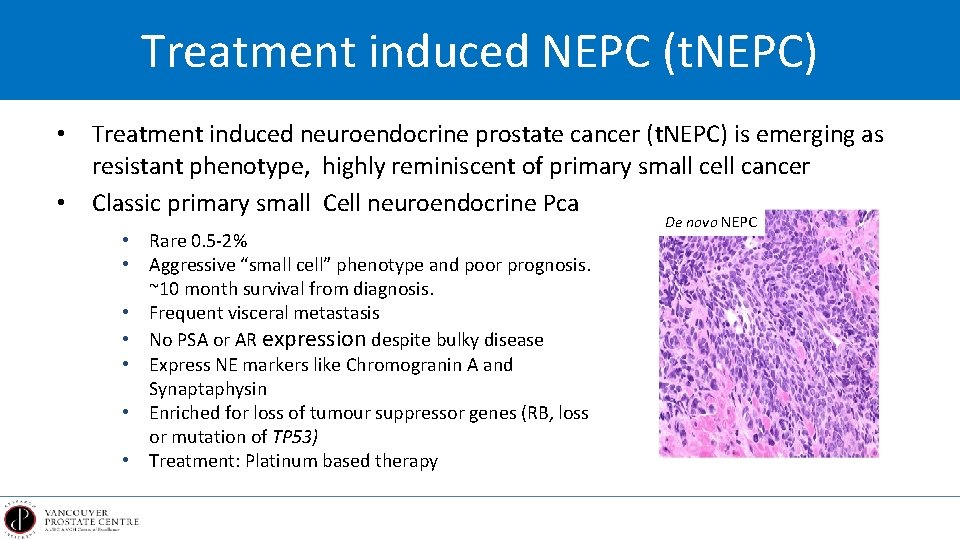
Treatment induced NEPC (t. NEPC) • Treatment induced neuroendocrine prostate cancer (t. NEPC) is emerging as resistant phenotype, highly reminiscent of primary small cell cancer • Classic primary small Cell neuroendocrine Pca • Rare 0. 5 -2% • Aggressive “small cell” phenotype and poor prognosis. ~10 month survival from diagnosis. • Frequent visceral metastasis • No PSA or AR expression despite bulky disease • Express NE markers like Chromogranin A and Synaptaphysin • Enriched for loss of tumour suppressor genes (RB, loss or mutation of TP 53) • Treatment: Platinum based therapy De novo NEPC

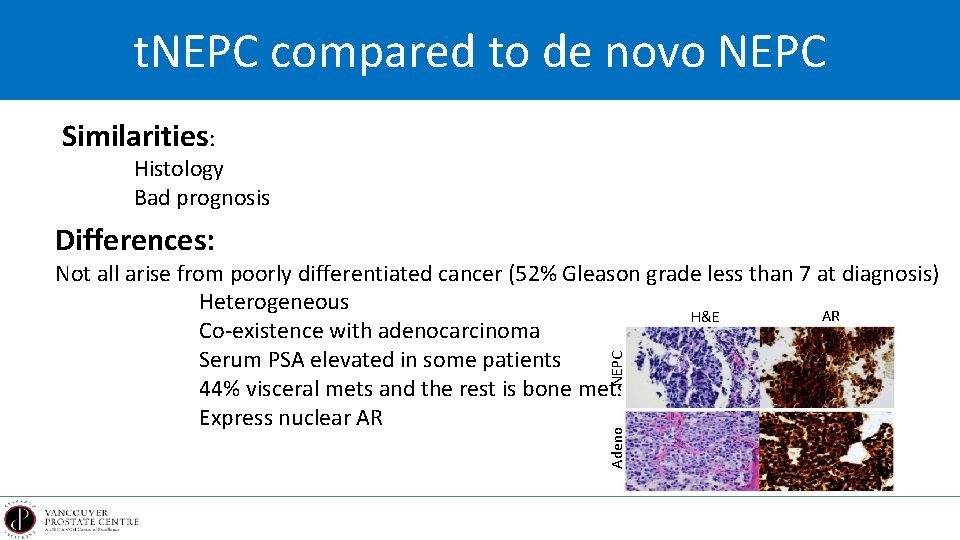
t. NEPC compared to de novo NEPC Similarities: Histology Bad prognosis Differences: Adeno NEPC Not all arise from poorly differentiated cancer (52% Gleason grade less than 7 at diagnosis) Heterogeneous AR H&E Co-existence with adenocarcinoma Serum PSA elevated in some patients 44% visceral mets and the rest is bone mets Express nuclear AR

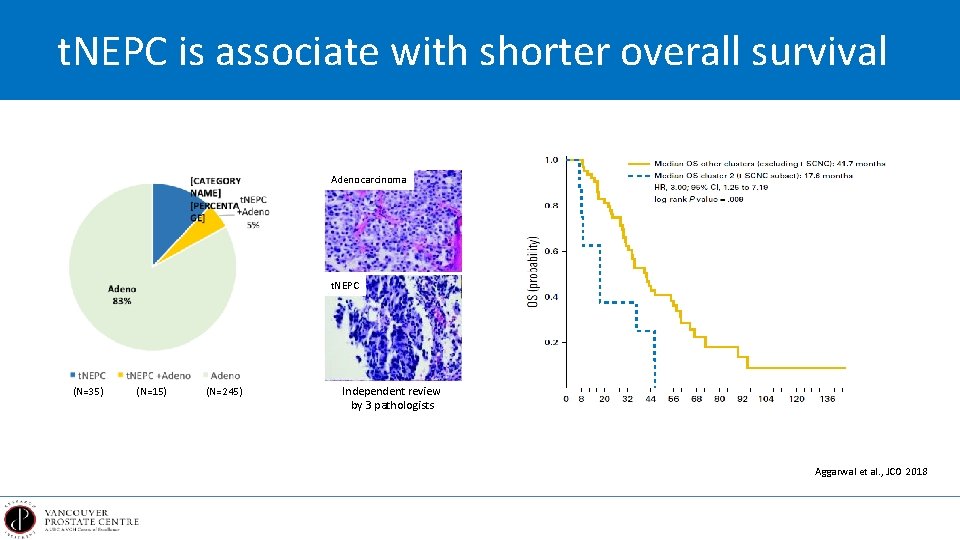
t. NEPC is associate with shorter overall survival Adenocarcinoma t. NEPC (N=35) (N=15) (N=245) Independent review by 3 pathologists Aggarwal et al. , JCO 2018

Questions What is driving t. NEPC? What are the potential therapeutic targets? How we can prevent the evolution of t. NEPC? What are the predictive biomarkers for t. NEPC?

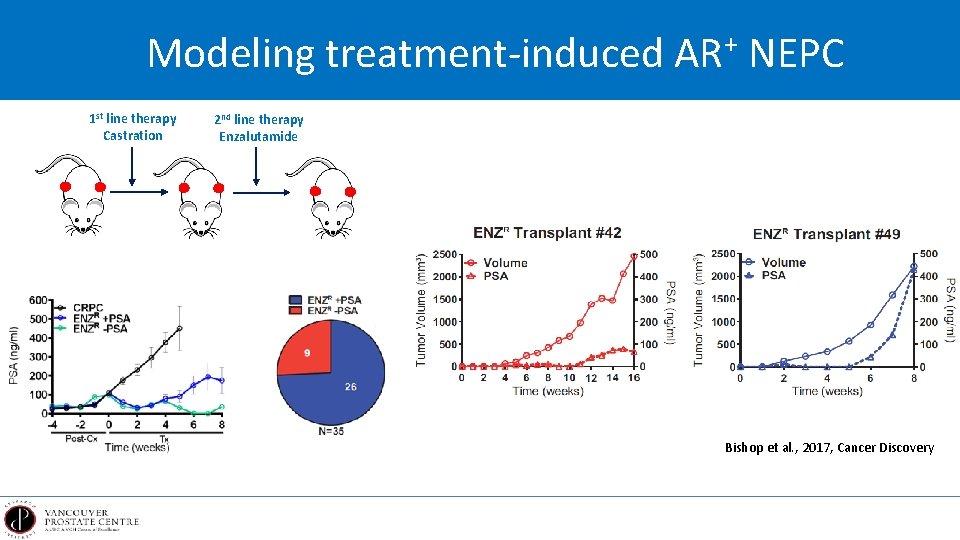
Modeling treatment-induced AR+ NEPC 1 st line therapy Castration 2 nd line therapy Enzalutamide Bishop et al. , 2017, Cancer Discovery

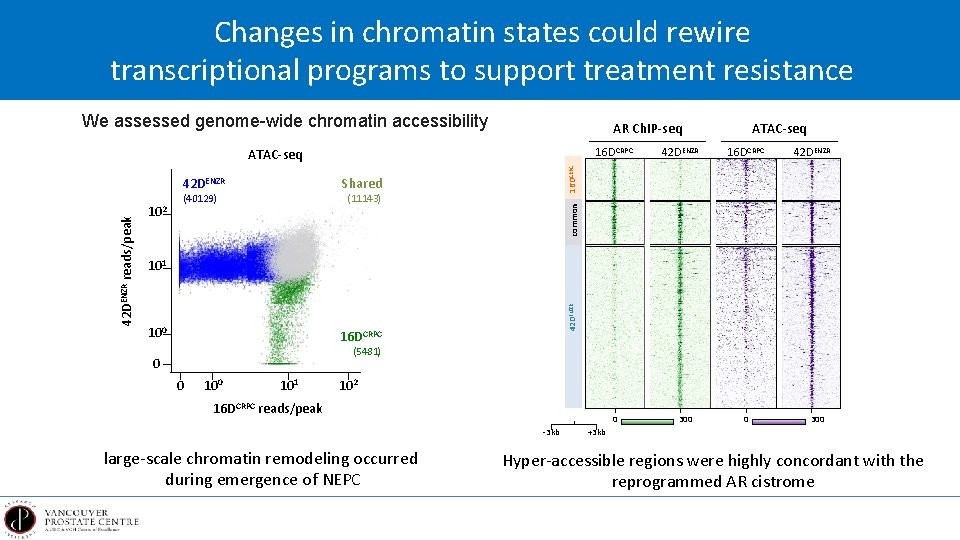
Changes in chromatin states could rewire transcriptional programs to support treatment resistance We assessed genome-wide chromatin accessibility AR Ch. IP-seq 16 DCRPC Shared (40129) 42 DENZR (11143) common 102 16 DCRPC 101 100 42 DENZR reads/peak 42 DENZR 16 DCRPC ATAC-seq 16 DCRPC (5481) 0 0 101 102 16 DCRPC reads/peak 0 -3 kb large-scale chromatin remodeling occurred during emergence of NEPC 300 0 300 +3 kb Hyper-accessible regions were highly concordant with the reprogrammed AR cistrome

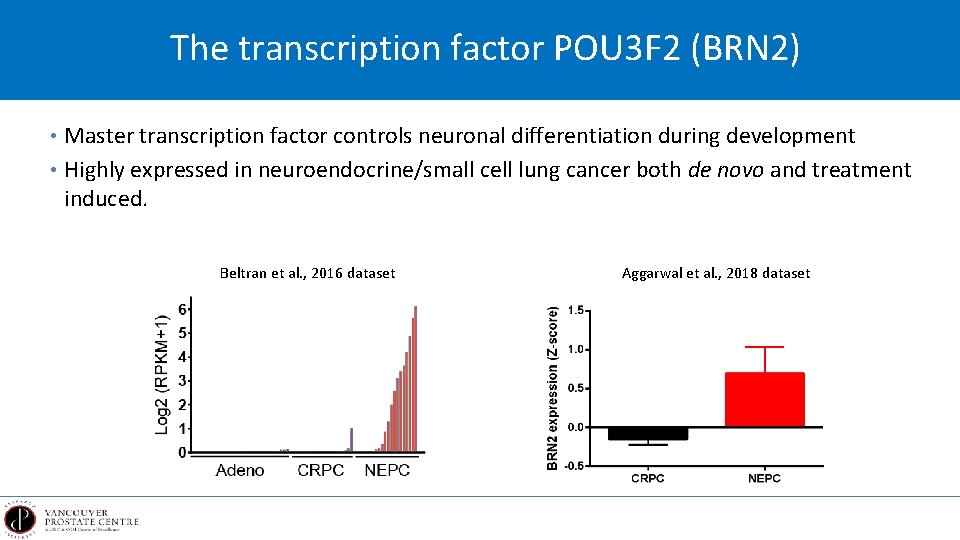
The transcription factor POU 3 F 2 (BRN 2) • Master transcription factor controls neuronal differentiation during development • Highly expressed in neuroendocrine/small cell lung cancer both de novo and treatment induced. Beltran et al. , 2016 dataset Aggarwal et al. , 2018 dataset

BRN 2: X-ray Crystallography

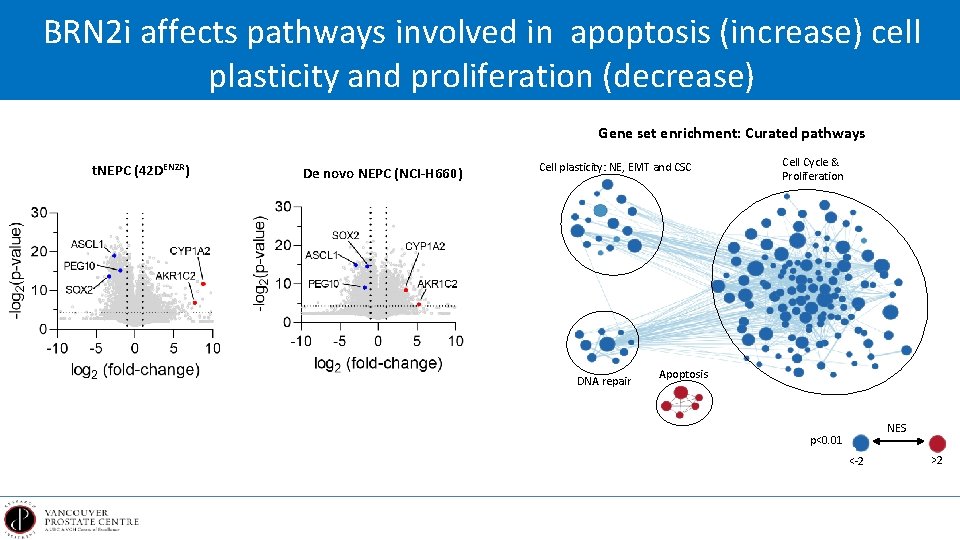
BRN 2 i affects pathways involved in apoptosis (increase) cell plasticity and proliferation (decrease) Gene set enrichment: Curated pathways t. NEPC (42 DENZR) De novo NEPC (NCI-H 660) Cell plasticity: NE, EMT and CSC DNA repair Cell Cycle & Proliferation Apoptosis NES p<0. 01 <-2 >2

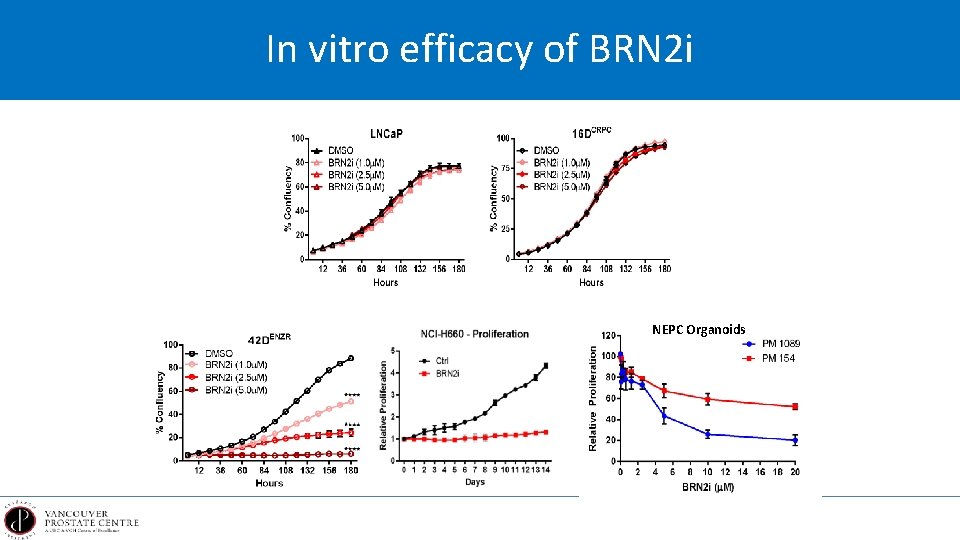
In vitro efficacy of BRN 2 i NEPC Organoids

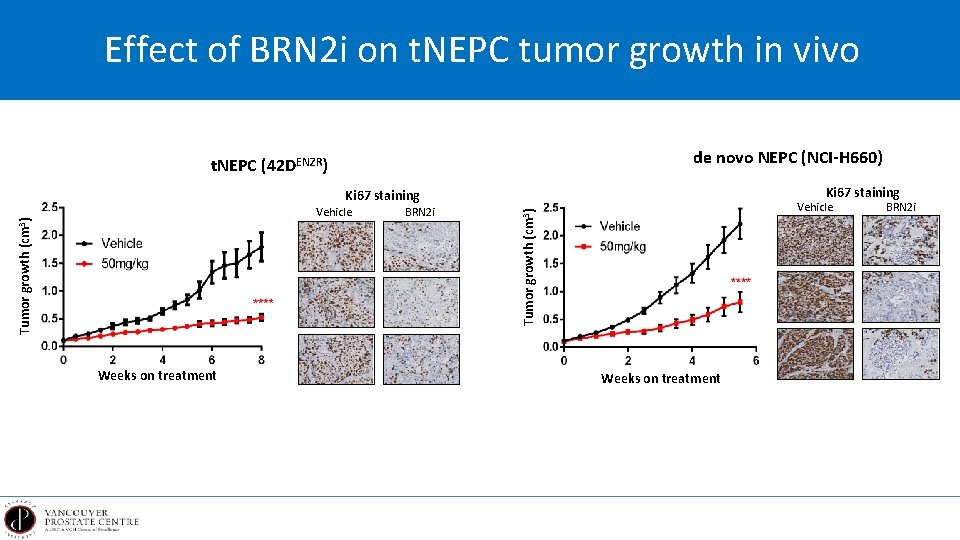
Effect of BRN 2 i on t. NEPC tumor growth in vivo de novo NEPC (NCI-H 660) t. NEPC (42 DENZR) Ki 67 staining Weeks on treatment BRN 2 i Tumor growth (cm 3) Vehicle Weeks on treatment BRN 2 i

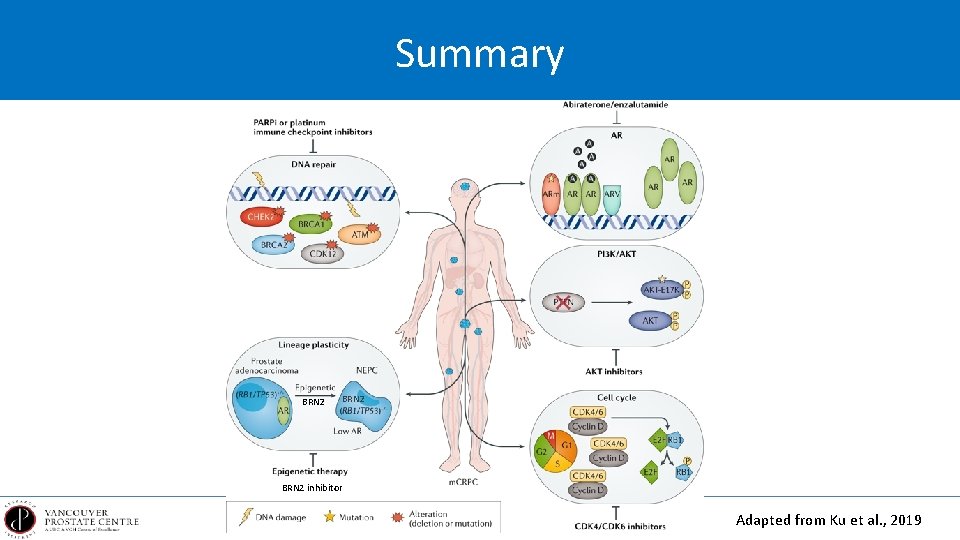
Summary BRN 2 inhibitor Adapted from Ku et al. , 2019

Acknowledgements Vancouver Prostate Centre Faraz Hach Dana Farber Cancer Institute Himisha Beltran University of Adelaide Luke Selth Wayne Tilley Princess Margaret Cancer Centre Hansen He Mayo Clinic Cancer Center Haojie Huang Roswell Park Cancer Institute David Goodrich