Table of Contents Chapter 4 Atomic Structure Subatomic

Table of Contents Chapter 4: Atomic Structure Subatomic Structure
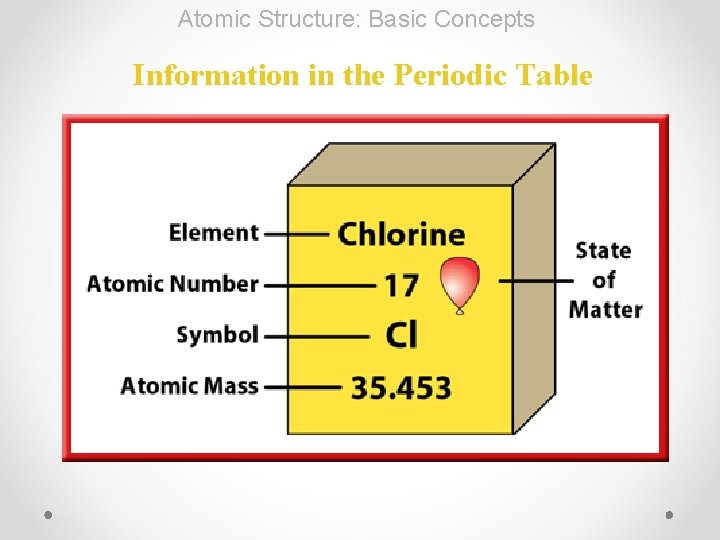
Atomic Structure: Basic Concepts Information in the Periodic Table
Subatomic Particles • Atoms have 3 subatomic particles Particle Abbrev. Grams a. m. u. Charge Protons P+ 1. 6 x 10 -24 1 +1 Neutrons N 0 1. 6 x 10 -24 1 0 Electrons e- 9. 11 x 10 -28 0 -1 • Protons & Neutrons make up the nucleus
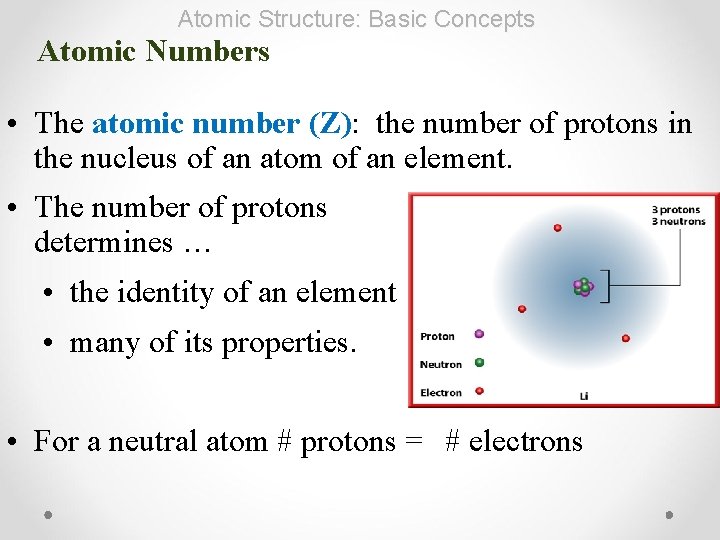
Atomic Structure: Basic Concepts Atomic Numbers • The atomic number (Z): the number of protons in the nucleus of an atom of an element. • The number of protons determines … • the identity of an element • many of its properties. • For a neutral atom # protons = # electrons


Atomic Mass (Mass Number) • The atomic mass (A): the total number of protons and neutrons in the nucleus of an atom • How do you figure out the # of neutrons?

Ions • Ions: an atom or group of atoms that has a positive or negative charge • Ions have a different number of electrons, not protons • Cation: A positive ion (electrons were lost) • Anion: A negative ion (electrons were gained)

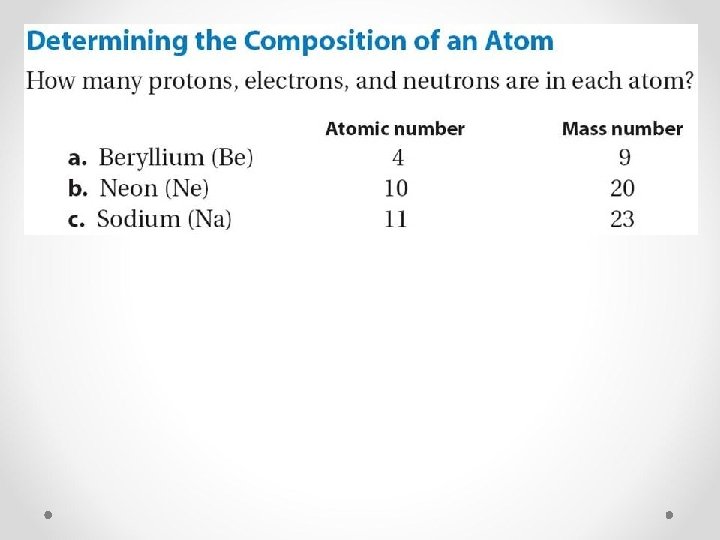
How many protons, electrons, and neutrons are in the following ions? Ca+2 K+1 Al+3 S-2 Br-1
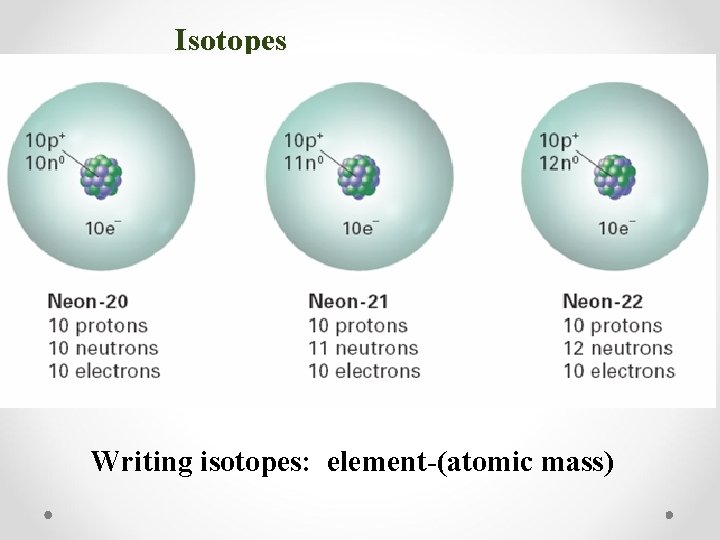
Isotopes • Isotopes: atoms with the same number of protons but different number of neutrons • Because they have different numbers of neutrons, they have different mass numbers • Despite these differences, isotopes are chemically alike because they have identical numbers of protons and electrons Writing isotopes: element-(atomic mass)

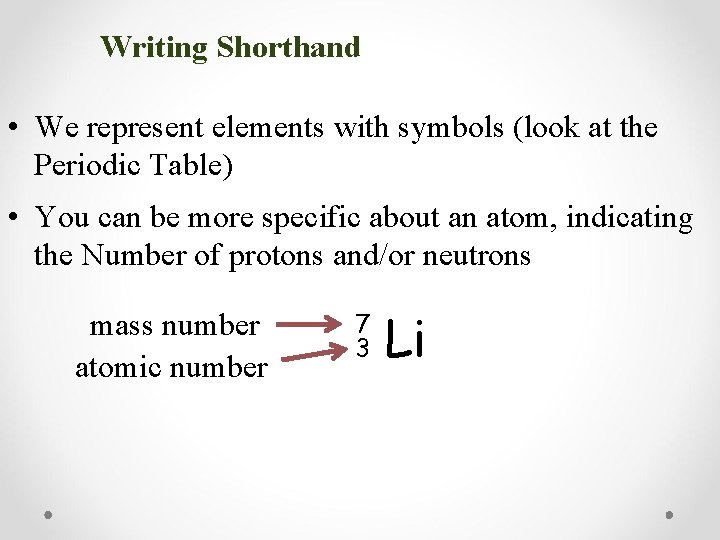
Writing Shorthand • We represent elements with symbols (look at the Periodic Table) • You can be more specific about an atom, indicating the Number of protons and/or neutrons mass number atomic number 7 3 Li
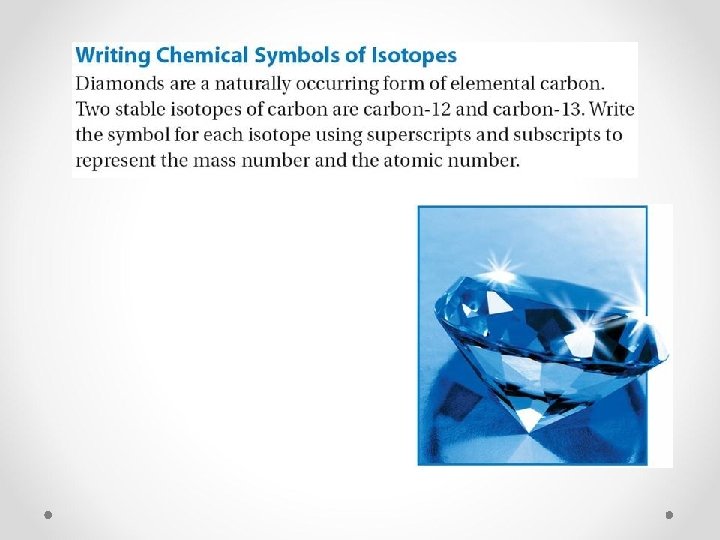
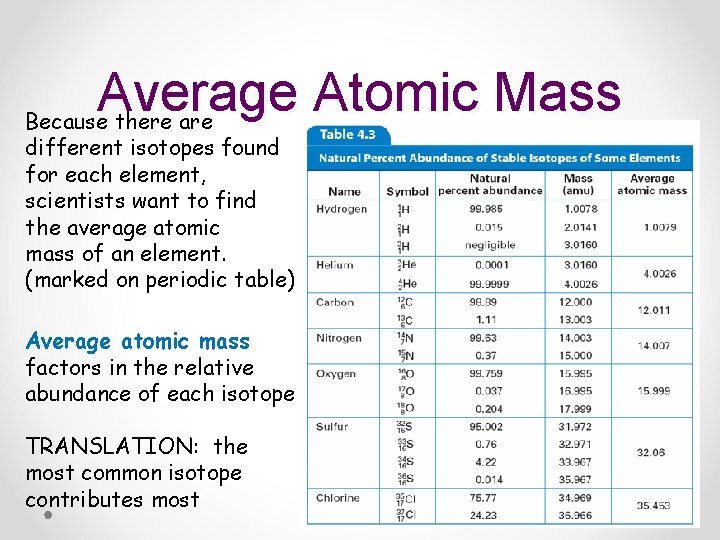
Average Atomic Mass Because there are different isotopes found for each element, scientists want to find the average atomic mass of an element. (marked on periodic table) Average atomic mass factors in the relative abundance of each isotope TRANSLATION: the most common isotope contributes most

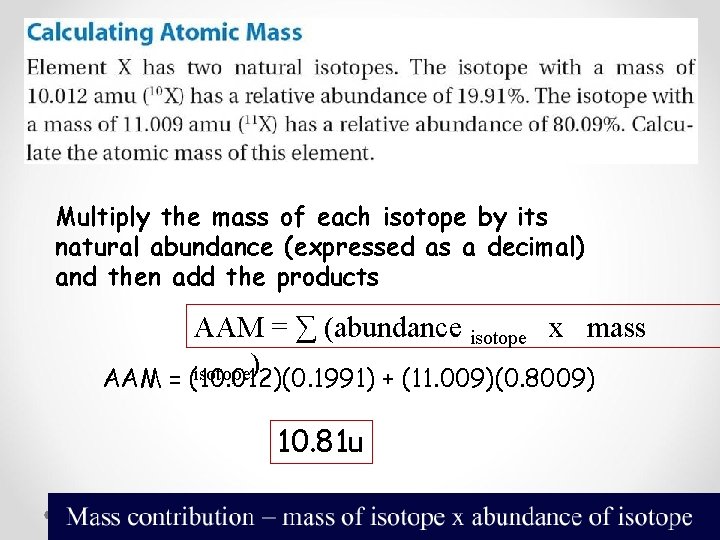
Multiply the mass of each isotope by its natural abundance (expressed as a decimal) and then add the products AAM = ∑ (abundance isotope x mass isotope) AAM = (10. 012)(0. 1991) + (11. 009)(0. 8009) 10. 81 u


Basic Concept Questions Question 1 How does the atomic number of an element differ from the element’s mass number? Answer
- Slides: 19