Table of Contents Chapter 20 OxidationReduction 20 2

Table of Contents Chapter 20: Oxidation-Reduction 20. 2: Balancing Redox Equations

Review • Define a “redox reaction” • Why do we use oxidation numbers? • What are the oxidation numbers for reach of the following elements in the following compound: PO 43 Cu 3 N 2 P=+5 O=-2 N=-3 Cu=+2
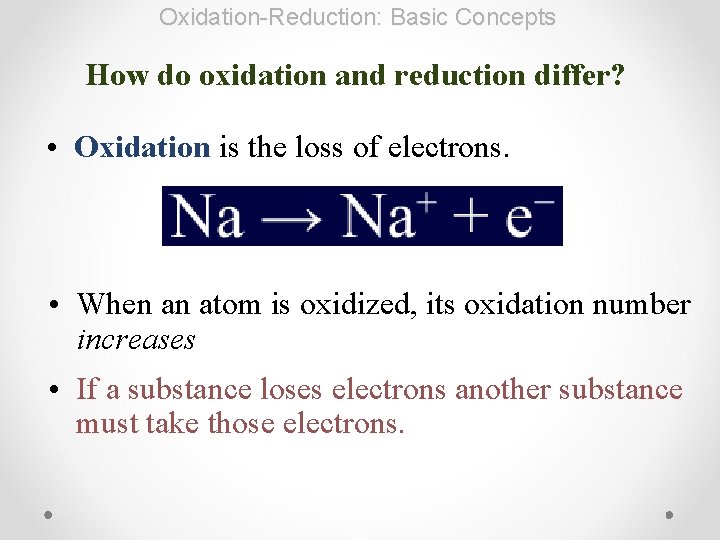
Oxidation-Reduction: Basic Concepts How do oxidation and reduction differ? • Oxidation is the loss of electrons. • When an atom is oxidized, its oxidation number increases • If a substance loses electrons another substance must take those electrons.
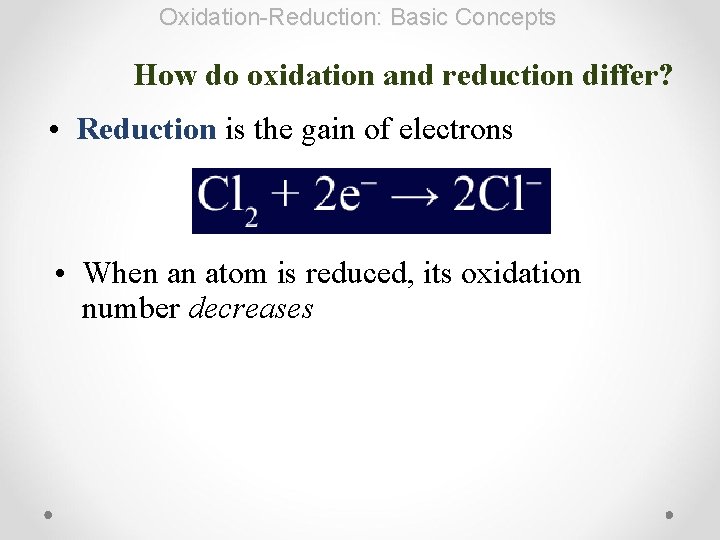
Oxidation-Reduction: Basic Concepts How do oxidation and reduction differ? • Reduction is the gain of electrons • When an atom is reduced, its oxidation number decreases
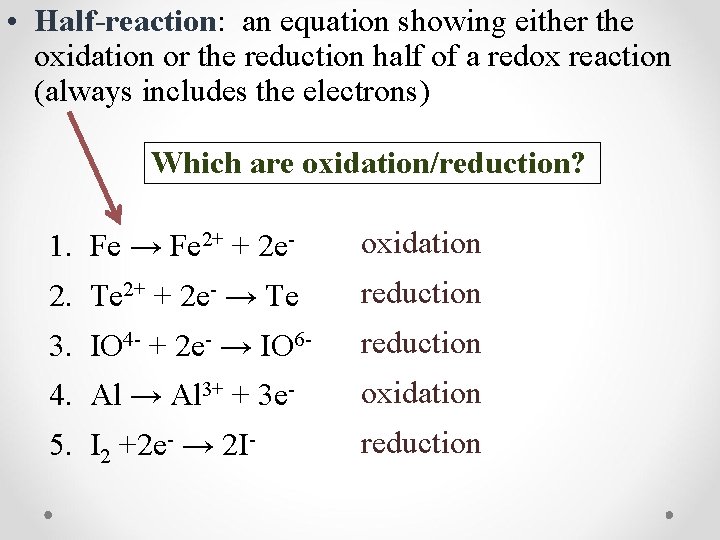
• Half-reaction: an equation showing either the oxidation or the reduction half of a redox reaction (always includes the electrons) Which are oxidation/reduction? 1. Fe → Fe 2+ + 2 e- oxidation 2. Te 2+ + 2 e- → Te reduction 3. IO 4 - + 2 e- → IO 6 - reduction 4. Al → Al 3+ + 3 e- oxidation 5. I 2 +2 e- → 2 I- reduction

Oxidation-Reduction: Basic Concepts How do oxidation and reduction differ? • Can oxidation occur without reduction? • Oxidation and reduction are complementary processes; oxidation cannot occur unless reduction also occurs.

Oxidation-Reduction: Basic Concepts How do oxidation and reduction differ? • LEO the lion says GER (LEO GER) • Loss of Electrons is Oxidation • Gain of Electrons is Reduction. • OIL RIG • Oxidation Is Loss • Reduction Is Gain
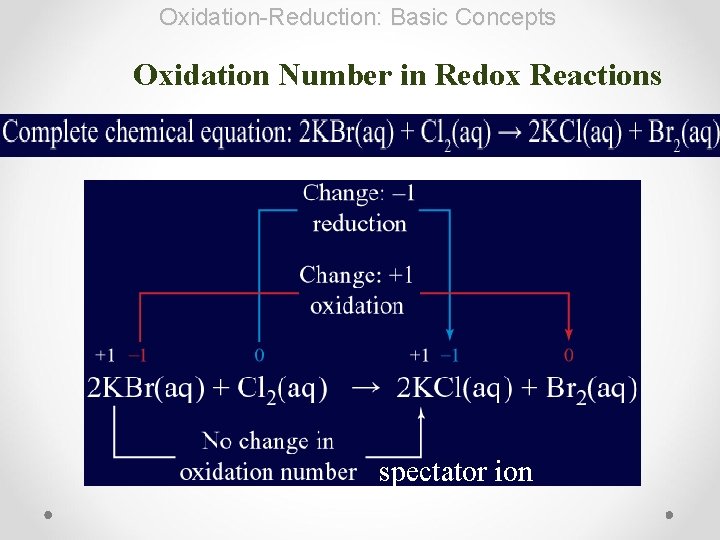
Oxidation-Reduction: Basic Concepts Oxidation Number in Redox Reactions spectator ion
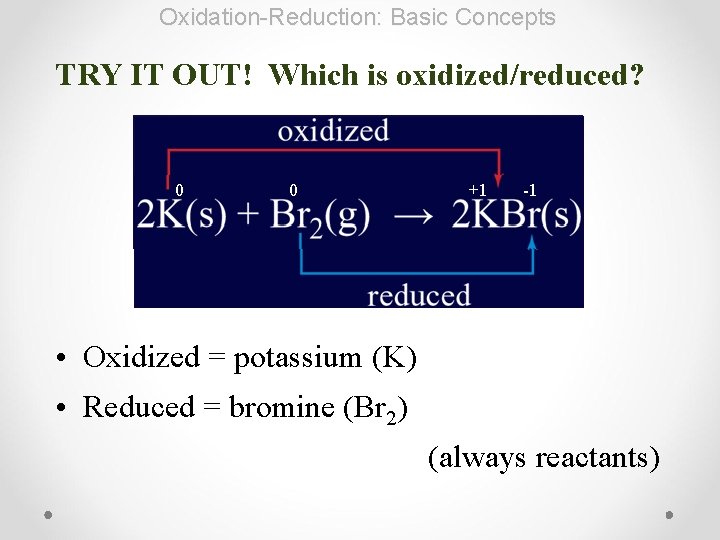
Oxidation-Reduction: Basic Concepts TRY IT OUT! Which is oxidized/reduced? 0 0 +1 -1 • Oxidized = potassium (K) • Reduced = bromine (Br 2) (always reactants)
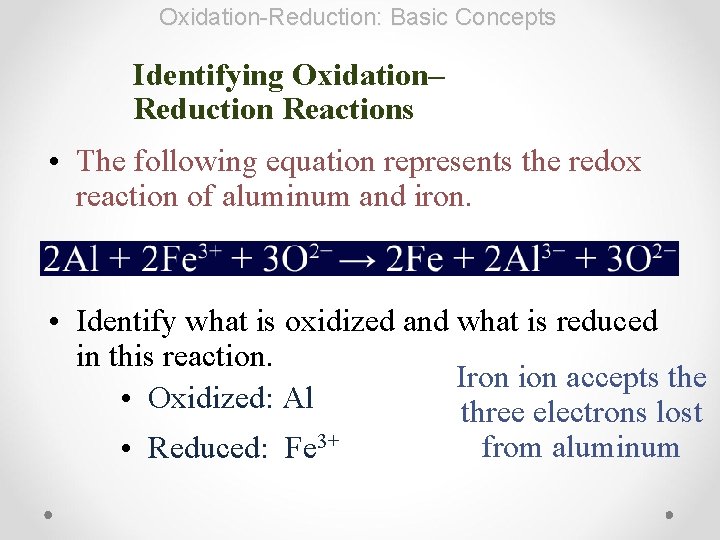
Oxidation-Reduction: Basic Concepts Identifying Oxidation– Reduction Reactions • The following equation represents the redox reaction of aluminum and iron. • Identify what is oxidized and what is reduced in this reaction. Iron ion accepts the • Oxidized: Al three electrons lost • Reduced: Fe 3+ from aluminum
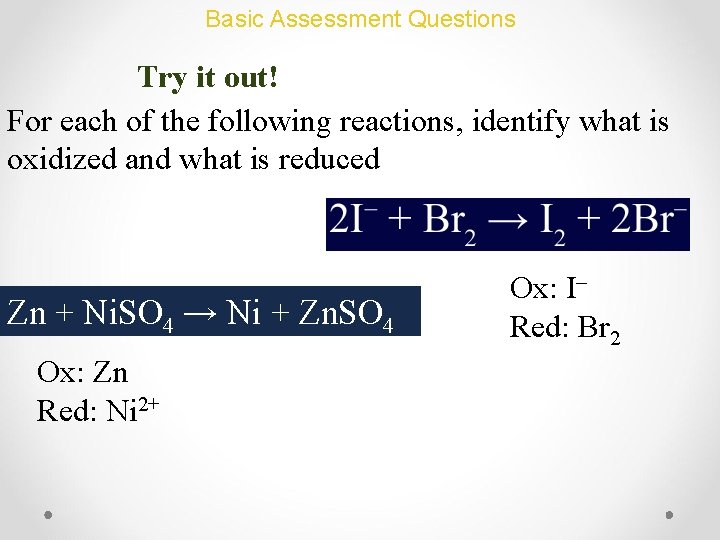
Basic Assessment Questions Try it out! For each of the following reactions, identify what is oxidized and what is reduced Zn + Ni. SO 4 → Ni + Zn. SO 4 Ox: Zn Red: Ni 2+ Ox: I– Red: Br 2
- Slides: 11