Table of Contents Chapter 18 Chemical Equilibrium 18

Table of Contents Chapter 18: Chemical Equilibrium 18. 3: Using equilibrium constants
Chemical Equilibrium: Basic Concepts Equilibrium Expressions and Constants • What is Keq? • Why do we use Keq? • Write Keq for the following reaction:
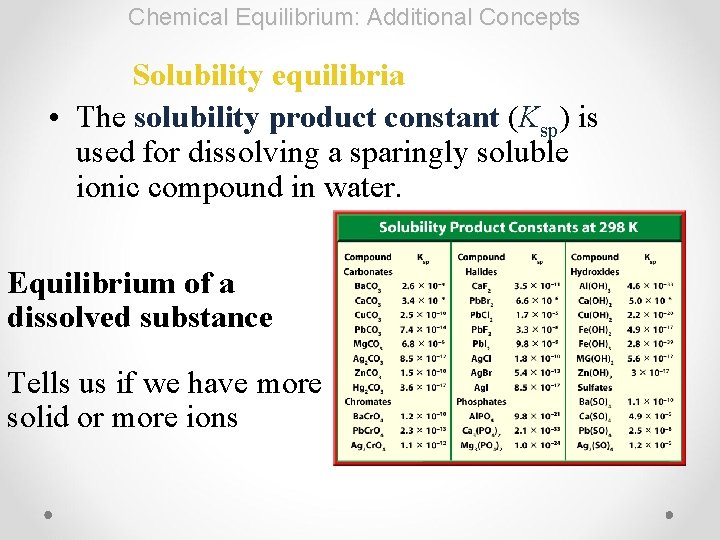
Chemical Equilibrium: Additional Concepts Solubility equilibria • The solubility product constant (Ksp) is used for dissolving a sparingly soluble ionic compound in water. Equilibrium of a dissolved substance Tells us if we have more solid or more ions

Chemical Equilibrium: Additional Concepts Solubility equilibria When you know Ksp, you can… 1. calculate the molar solubility of a sparingly soluble ionic compound 2. calculate moles per liter of a saturated substance. 3. calculate ion concentrations in a saturated solution. These all use the same process!
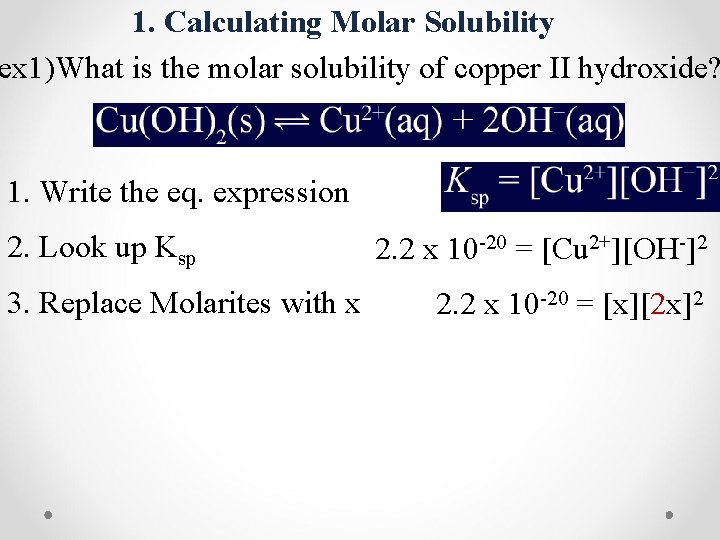
1. Calculating Molar Solubility ex 1)What is the molar solubility of copper II hydroxide? 1. Write the eq. expression 2. Look up Ksp 3. Replace Molarites with x 2. 2 x 10 -20 = [Cu 2+][OH-]2 2. 2 x 10 -20 = [x][2 x]2

1. Calculating Molar Solubility ex 1)What is the molar solubility of copper II hydroxide? 4. Solve for x 2. 2 x 10 -20 = [x][2 x]2 = x 4 x 2 = 4 x 3 [x]3 = 5. 5 x 10 -21 [x] = 1. 8 x 10 -7 M The solubility of Cu(OH)2 is = 1. 8 x 10 -7 mol/L
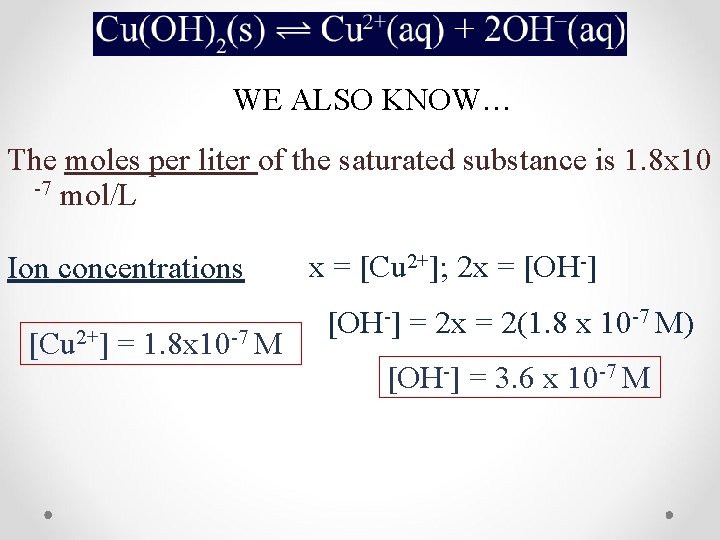
WE ALSO KNOW… The moles per liter of the saturated substance is 1. 8 x 10 -7 mol/L Ion concentrations [Cu 2+] = 1. 8 x 10 -7 M x = [Cu 2+]; 2 x = [OH-] = 2 x = 2(1. 8 x 10 -7 M) [OH-] = 3. 6 x 10 -7 M
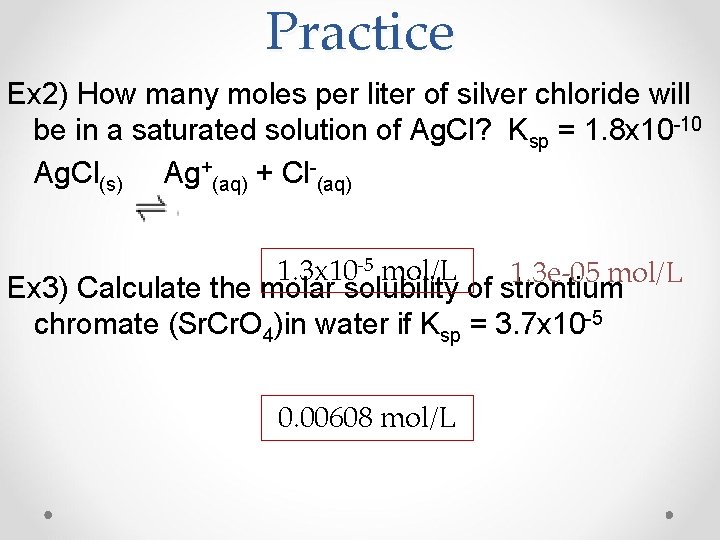
Practice Ex 2) How many moles per liter of silver chloride will be in a saturated solution of Ag. Cl? Ksp = 1. 8 x 10 -10 Ag. Cl(s) Ag+(aq) + Cl-(aq) 1. 3 x 10 -5 mol/L 1. 3 e-05 mol/L Ex 3) Calculate the molar solubility of strontium chromate (Sr. Cr. O 4)in water if Ksp = 3. 7 x 10 -5 0. 00608 mol/L
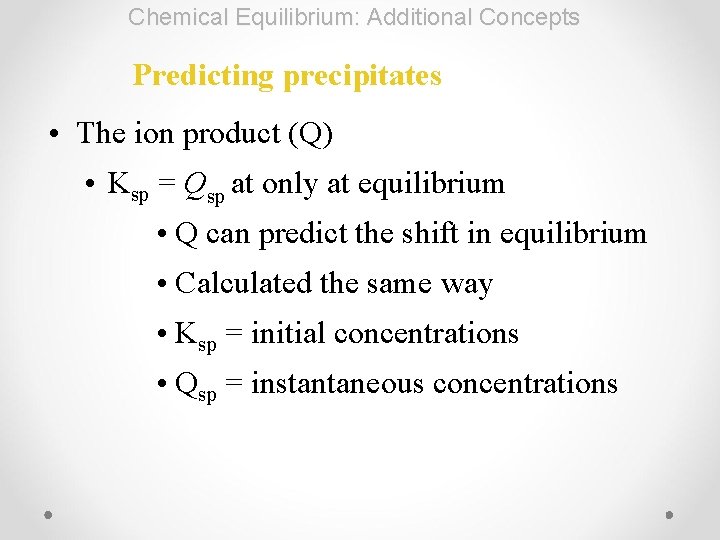
Chemical Equilibrium: Additional Concepts Predicting precipitates • The ion product (Q) • Ksp = Qsp at only at equilibrium • Q can predict the shift in equilibrium • Calculated the same way • Ksp = initial concentrations • Qsp = instantaneous concentrations

Chemical Equilibrium: Additional Concepts Predicting precipitates • If Qsp < Ksp, shift to reactants, no precipitate forms • If Qsp > Ksp, shift to products, precipitate will form • If Qsp = Ksp, no change will occur

Q ex 4) Predict whether a precipitate of Pb. Cl 2 will form if 1 L of 0. 01 M Na. Cl is added to 1 L of 0. 2 M Pb(NO 3)2. Ksp = 1. 7 x 10 -5 How did they know which precipitate? Solubility rules! Back of the periodic table
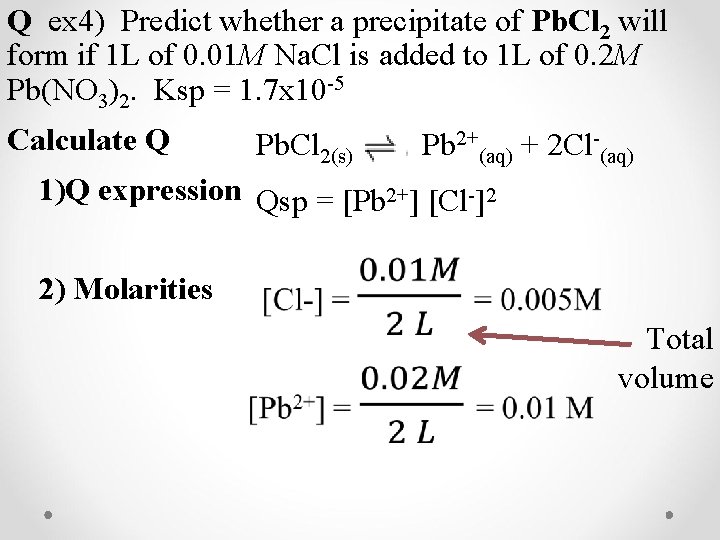
Q ex 4) Predict whether a precipitate of Pb. Cl 2 will form if 1 L of 0. 01 M Na. Cl is added to 1 L of 0. 2 M Pb(NO 3)2. Ksp = 1. 7 x 10 -5 Calculate Q Pb. Cl 2(s) Pb 2+(aq) + 2 Cl-(aq) 1)Q expression Qsp = [Pb 2+] [Cl-]2 2) Molarities Total volume
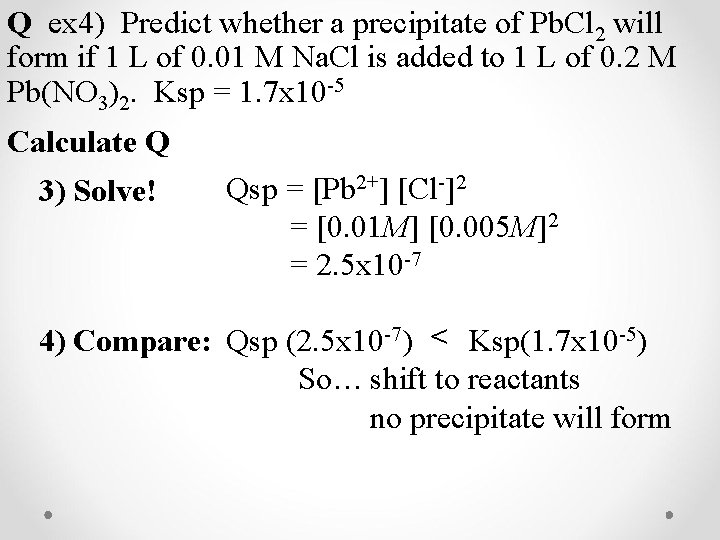
Q ex 4) Predict whether a precipitate of Pb. Cl 2 will form if 1 L of 0. 01 M Na. Cl is added to 1 L of 0. 2 M Pb(NO 3)2. Ksp = 1. 7 x 10 -5 Calculate Q 3) Solve! Qsp = [Pb 2+] [Cl-]2 = [0. 01 M] [0. 005 M]2 = 2. 5 x 10 -7 4) Compare: Qsp (2. 5 x 10 -7) < Ksp(1. 7 x 10 -5) So… shift to reactants no precipitate will form
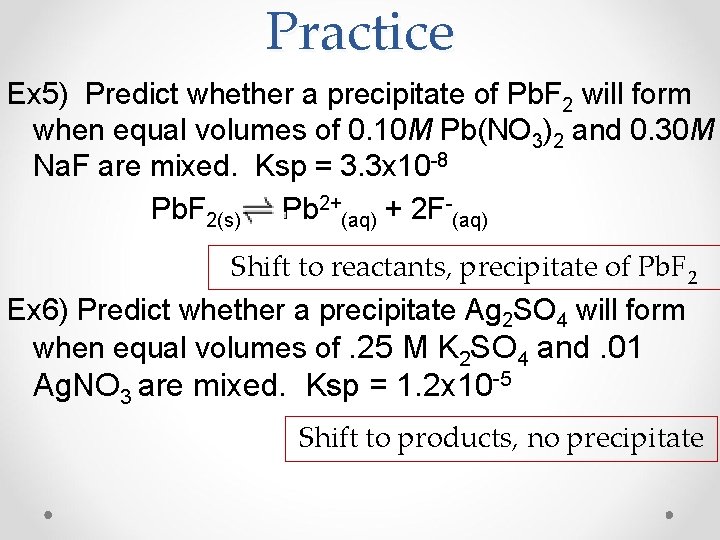
Practice Ex 5) Predict whether a precipitate of Pb. F 2 will form when equal volumes of 0. 10 M Pb(NO 3)2 and 0. 30 M Na. F are mixed. Ksp = 3. 3 x 10 -8 Pb. F 2(s) Pb 2+(aq) + 2 F-(aq) Shift to reactants, precipitate of Pb. F 2 Ex 6) Predict whether a precipitate Ag 2 SO 4 will form when equal volumes of. 25 M K 2 SO 4 and. 01 Ag. NO 3 are mixed. Ksp = 1. 2 x 10 -5 Shift to products, no precipitate

Chemical Equilibrium: Additional Concepts Common ion effect • Common ion effect: The solubility of a substance is reduced when the substance is dissolved in a solution containing a common ion. • For example, Pb. I 2 is less soluble in an aqueous solution of Na. I than in pure water. • Because the common ion I– is already present in the Na. I solution. It reduces the maximum possible concentration of Pb 2+ and thus reduces the solubility of Pb. I 2.
- Slides: 15