Table of Contents Chapter 12 Stoichiometry 12 1

Table of Contents Chapter 12: Stoichiometry 12. 1: What is Stoichiometry? 12. 2: Stoichiometric Calculations
Table of Contents Chapter 12: Stoichiometry • Stoichiometry is the mathematical study of chemical reactions. 12. 1: Mole-Mass • Stoichiometry ratios • The molar ratio of one reactant/product to 12. 2: Stoichiometric Calculations another reactant/product • Used to calculate how much reactant (or product) will be consumed (or created) in a chemical reaction. • It shows quantitative relationship between the reactants and products.
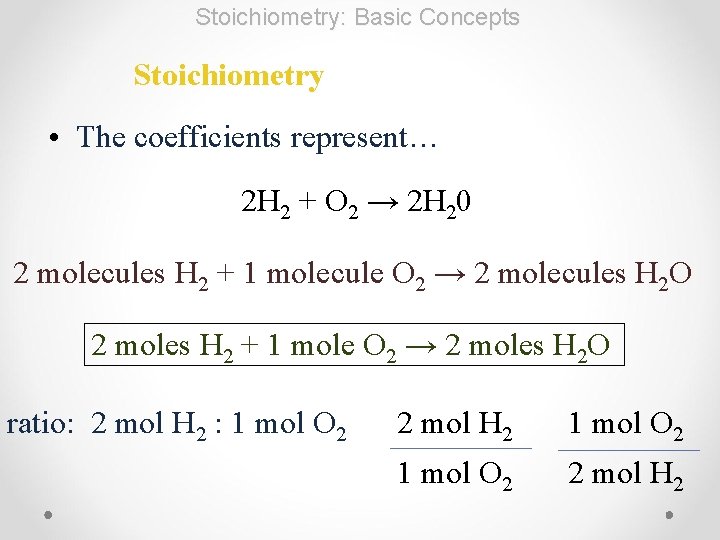
Stoichiometry: Basic Concepts Stoichiometry • The coefficients represent… 2 H 2 + O 2 → 2 H 20 2 molecules H 2 + 1 molecule O 2 → 2 molecules H 2 O 2 moles H 2 + 1 mole O 2 → 2 moles H 2 O ratio: 2 mol H 2 : 1 mol O 2 2 mol H 2

Stoichiometry: Basic Concepts Stoichiometry problems 1. Write balanced equation a. Identify reactants and products b. Write their chemical formulas (balance charges) c. Balance the equation

Stoichiometry: Basic Concepts Stoichiometry problems 2. Convert to moles Just like we’ve been practicing 3. Convert from moles known to moles of unknown (Apply the mole to mole ratio from the balanced equation) 4. Convert moles to needed units
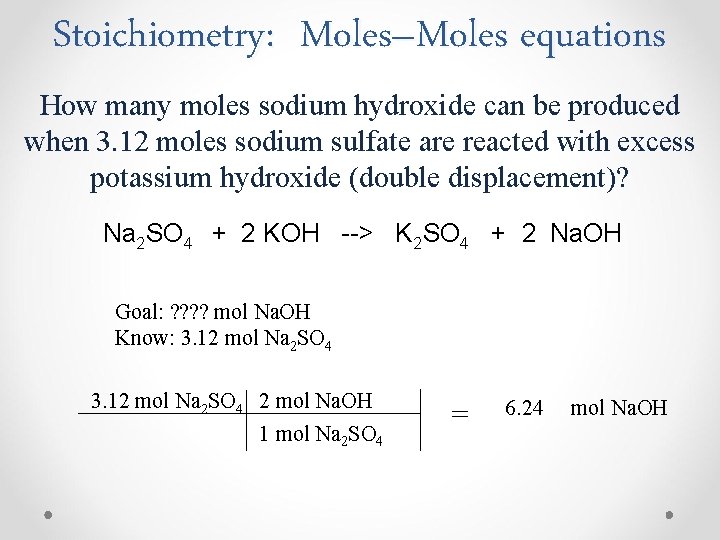
Stoichiometry: Moles–Moles equations How many moles sodium hydroxide can be produced when 3. 12 moles sodium sulfate are reacted with excess potassium hydroxide (double displacement)? Na 2 SO 4 + 2 KOH --> K 2 SO 4 + 2 Na. OH Goal: ? ? mol Na. OH Know: 3. 12 mol Na 2 SO 4 2 mol Na. OH 1 mol Na 2 SO 4 = 6. 24 mol Na. OH
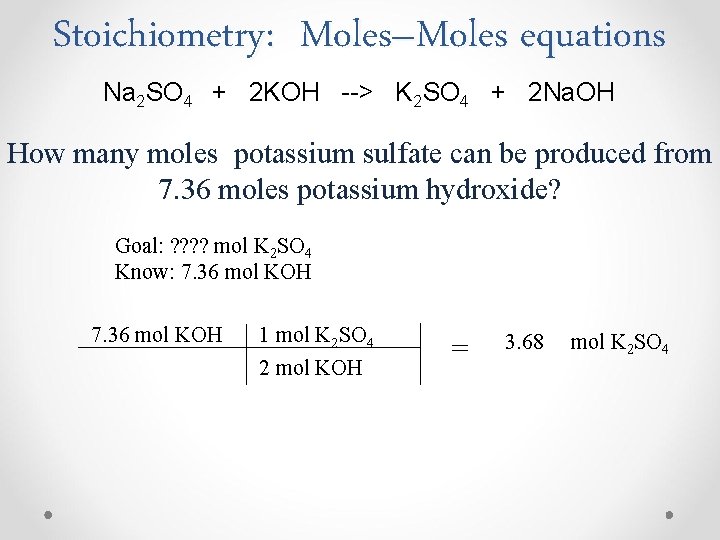
Stoichiometry: Moles–Moles equations Na 2 SO 4 + 2 KOH --> K 2 SO 4 + 2 Na. OH How many moles potassium sulfate can be produced from 7. 36 moles potassium hydroxide? Goal: ? ? mol K 2 SO 4 Know: 7. 36 mol KOH 1 mol K 2 SO 4 2 mol KOH = 3. 68 mol K 2 SO 4
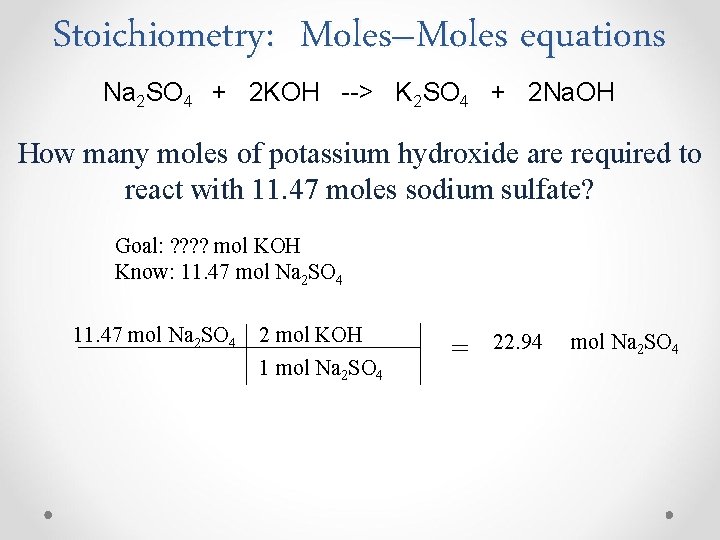
Stoichiometry: Moles–Moles equations Na 2 SO 4 + 2 KOH --> K 2 SO 4 + 2 Na. OH How many moles of potassium hydroxide are required to react with 11. 47 moles sodium sulfate? Goal: ? ? mol KOH Know: 11. 47 mol Na 2 SO 4 2 mol KOH 1 mol Na 2 SO 4 = 22. 94 mol Na 2 SO 4
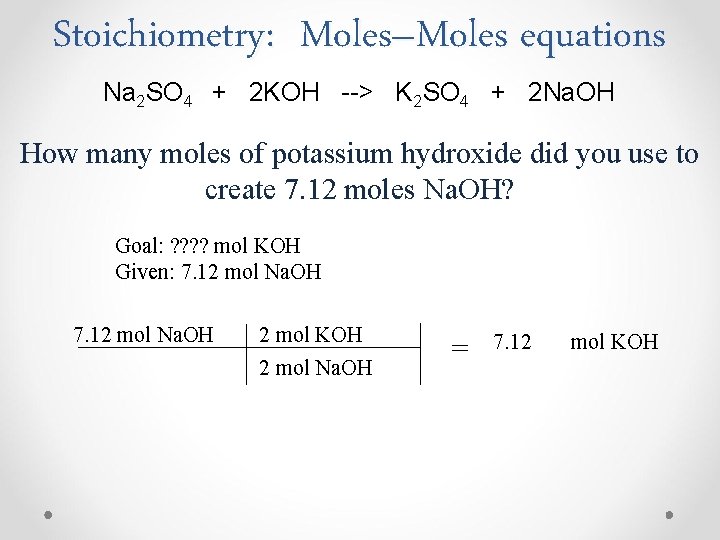
Stoichiometry: Moles–Moles equations Na 2 SO 4 + 2 KOH --> K 2 SO 4 + 2 Na. OH How many moles of potassium hydroxide did you use to create 7. 12 moles Na. OH? Goal: ? ? mol KOH Given: 7. 12 mol Na. OH 2 mol KOH 2 mol Na. OH = 7. 12 mol KOH
Stoichiometry: Basic Concepts Stoichiometry: Mass-Mass problems Mole - mole Mass – mole / mole - mass
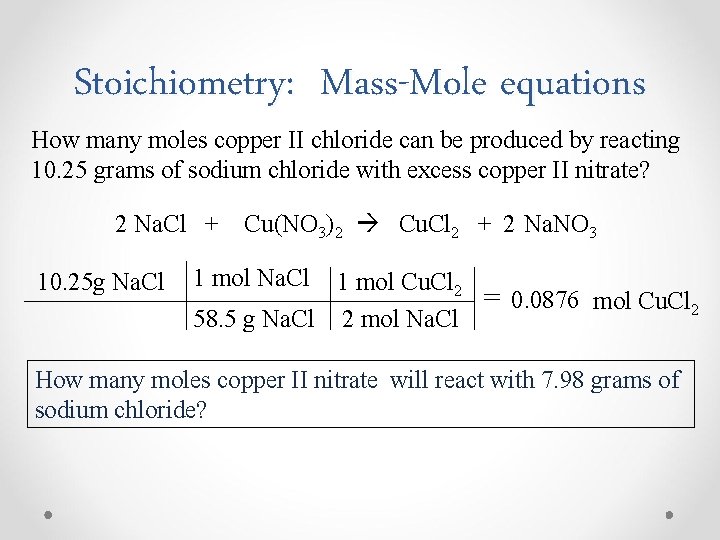
Stoichiometry: Mass-Mole equations How many moles copper II chloride can be produced by reacting 10. 25 grams of sodium chloride with excess copper II nitrate? 2 Na. Cl + 10. 25 g Na. Cl Cu(NO 3)2 Cu. Cl 2 + 2 Na. NO 3 1 mol Na. Cl 1 mol Cu. Cl 2 58. 5 g Na. Cl 2 mol Na. Cl = 0. 0876 mol Cu. Cl 2 How many moles copper II nitrate will react with 7. 98 grams of sodium chloride?
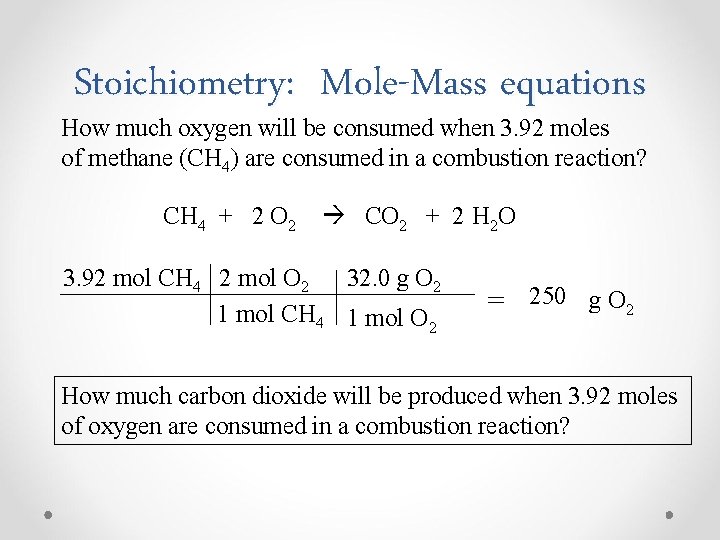
Stoichiometry: Mole-Mass equations How much oxygen will be consumed when 3. 92 moles of methane (CH 4) are consumed in a combustion reaction? CH 4 + 2 O 2 CO 2 + 2 H 2 O 3. 92 mol CH 4 2 mol O 2 32. 0 g O 2 1 mol CH 4 1 mol O 2 = 250 g O 2 How much carbon dioxide will be produced when 3. 92 moles of oxygen are consumed in a combustion reaction?
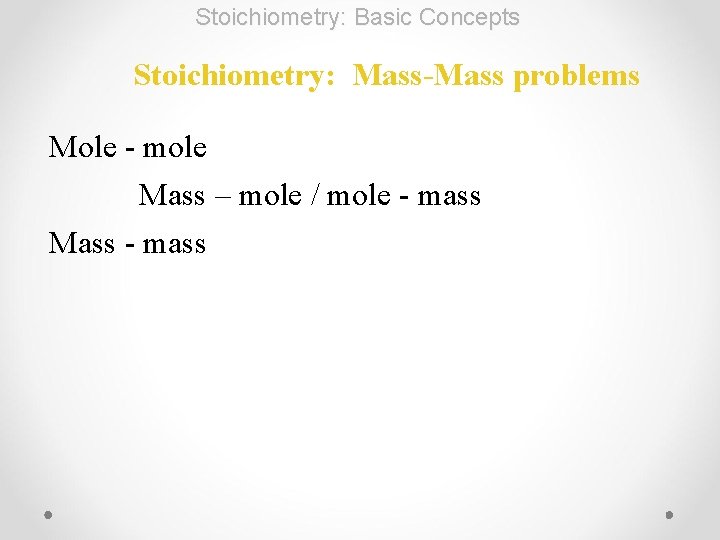
Stoichiometry: Basic Concepts Stoichiometry: Mass-Mass problems Mole - mole Mass – mole / mole - mass Mass - mass
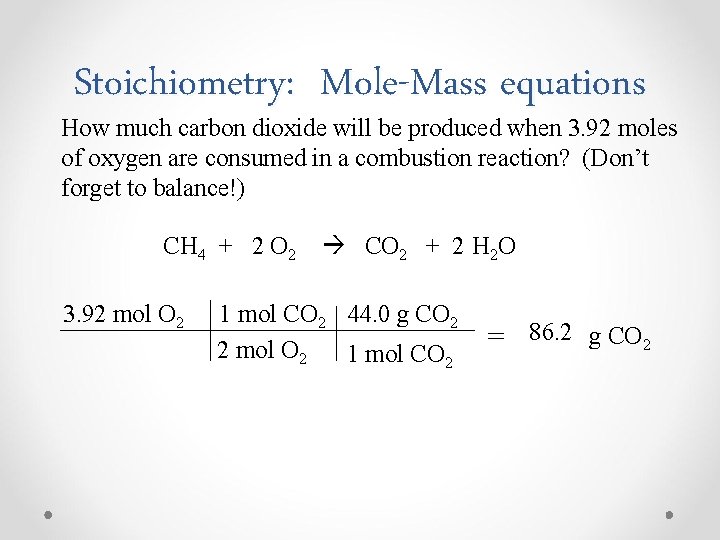
Stoichiometry: Mole-Mass equations How much carbon dioxide will be produced when 3. 92 moles of oxygen are consumed in a combustion reaction? (Don’t forget to balance!) CH 4 + 2 O 2 CO 2 + 2 H 2 O 3. 92 mol O 2 1 mol CO 2 44. 0 g CO 2 2 mol O 2 1 mol CO 2 = 86. 2 g CO 2

Stoichiometry: Mass-Mass equations How many grams copper II chloride can be produced by reacting 10. 25 grams of sodium chloride with excess copper II nitrate? (Double Displacement Reaction) 2 Na. Cl + Cu(NO 3)2 Cu. Cl 2 + 2 Na. NO 3 Given: 10. 25 g Na. Cl Want: ? ? Cu. Cl 2 10. 25 g Na. Cl 1 mol Cu. Cl 2 134. 5 g Cu. Cl 2 58. 5 g Na. Cl 2 mol Na. Cl 1 mol Cu. Cl 2 = 11. 8 g Cu. Cl 2
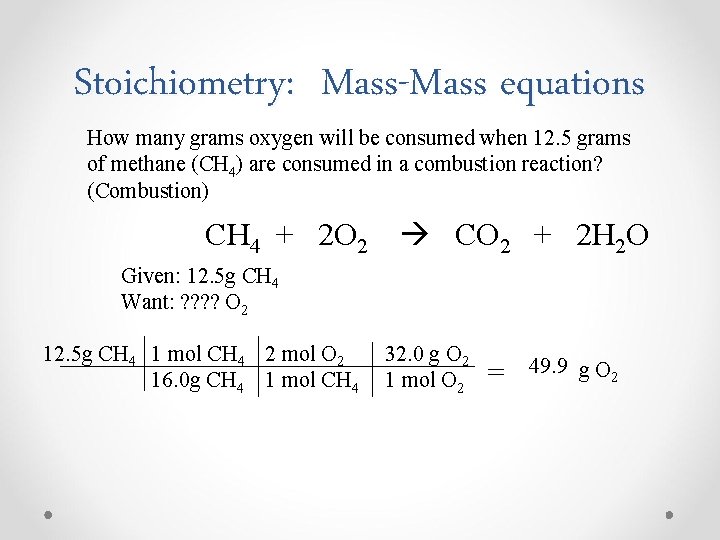
Stoichiometry: Mass-Mass equations How many grams oxygen will be consumed when 12. 5 grams of methane (CH 4) are consumed in a combustion reaction? (Combustion) CH 4 + 2 O 2 CO 2 + 2 H 2 O Given: 12. 5 g CH 4 Want: ? ? O 2 12. 5 g CH 4 1 mol CH 4 2 mol O 2 16. 0 g CH 4 1 mol CH 4 32. 0 g O 2 1 mol O 2 = 49. 9 g O 2

End Ch. 12. 2
- Slides: 17